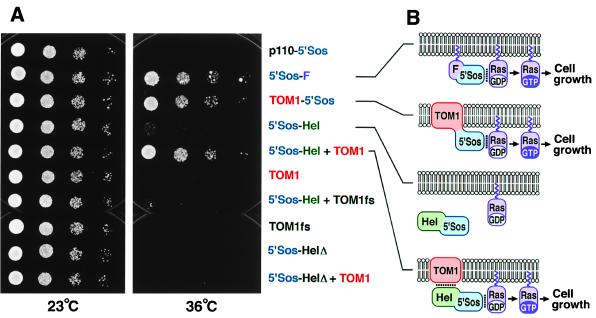
Figure 5.
Interaction between TOM1 and the TMV-Cg-encoded replication proteins. (A) cdc25–2 yeast strains harboring plasmids designed to constitutively express indicated proteins were diluted in sterile water to absorbance at 600 nm of 0.2, 0.025, 0.003, and 0.0004 (8-fold serial dilutions). Each dilution (2 μl) was spotted onto YAPD plates [1% (wt/vol) yeast extract/2% (wt/vol) peptone/2% (wt/vol) glucose/0.004% (wt/vol) adenine hemisulfate/2% (wt/vol) agar] and cultured at 23°C for 52 h or 36°C for 66 h. Part of p110 β, a subunit of phosphatidylinositol-3-phosphate kinase, fused to 5′Sos (p110–5′Sos), and 5′Sos fused to Ras farnesylation signal (5′Sos-F) are negative and positive controls, respectively, for suppression of cdc25–2 temperature sensitivity (24). TOM1fs and 5′Sos-HelΔ are frameshifting and deletion derivatives of TOM1 and 5′Sos-Hel, respectively. (B) Models explaining the results of A. Lipid bilayers indicate plasma membranes with the lower sides cytoplasmic. Noncovalent interactions are indicated by dotted lines. Covalent linkage of 5′Sos polypeptide with TOM1 or a noncovalent interaction between TOM1 and the helicase domain of TMV-Cg-encoded replication proteins in 5′Sos-Hel recruits 5′Sos to the plasma membrane to activate Ras signaling.