Table 1.
Structures and inhibitory activities of compounds in the training set.
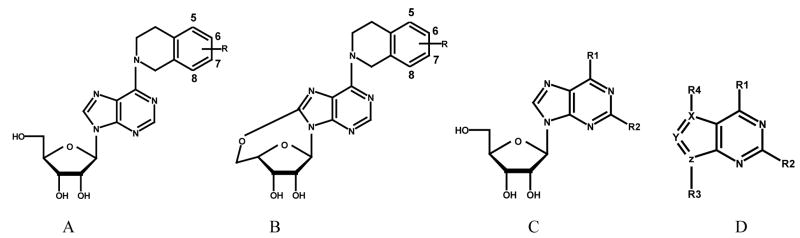 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd | Type | R | R1 | R2 | R3 | R4 | X | Y | Z | pIC50 |
| 1, NBMPR | C | -S- (4-nitrobenzyl) | H | 8.07a | ||||||
| 2 | A | 5-NO2 | 5.520a | |||||||
| 3 | A | 6-NO2 | 6.740a | |||||||
| 4 | A | 7-NO2 | 8.264a | |||||||
| 5 | A | 8-NO2 | 5.440a | |||||||
| 6 | B | 5-NO2 | 2.130a | |||||||
| 7 | B | 6-NO2 | 3.610a | |||||||
| 8 | B | 7-NO2 | 6.480a | |||||||
| 9 | B | 8-NO2 | 2.76a | |||||||
| 26 | A | H | 5.740a | |||||||
| 27 | C | -S- (4-nitrobenzyl) | NH2 | 7.670b | ||||||
| 28 | C | -NH-n-amyl | H | 4.870b | ||||||
| 29 | C | -NH-benzyl | H | 4.970b | ||||||
| 30 | C | NH-2-ethoxyethyladenosine | H | 3.04b | ||||||
| 31 | C | -NH-furfuryl | H | 4.44b | ||||||
| 32 | -NH-isopropyl | H | 3.59b | |||||||
| 33 | C | -N(CH3)-(4-nitrobenzyl) | H | 6.51b | ||||||
| 34 | C | - NH- (4-nitrobenzyl) | H | 7.56b | ||||||
| 35 | C | -NH-phenyl | H | 4.49b | ||||||
| 36 | C | -NH-2-thenyl | H | 4.75b | ||||||
| 37 | C | -O-benzyl | H | 5.68b | ||||||
| 38 | C | -S-allyl | H | 4.86b | ||||||
| 39 | C | -S-benzyl | H | 5.77b | ||||||
| 40 | C | -S-cyclohexylmethyl | H | 5.81b | ||||||
| 41 | C | -S-cyclohexyl | H | 5.20b | ||||||
| 42 | C | -S-α, α-dimethylbenzyl | H | 4.67b | ||||||
| 43 | C | -S-ethyl | H | 4.24b | ||||||
| 44 | C | -S- (2-hydroxy-5-nitro-benzyl) | H | 7.48b | ||||||
| 45 | C | -S- (4-isopropylbenzyl) | H | 5.32b | ||||||
| 46 | C | -S- [(2-methyl-1-naphthyl)-methyl] | H | 3.94b | ||||||
| 47 | C | -S-methyl | H | 3.64b | ||||||
| 48 | D | -S-methyl | H | tetrahydropyran-2-yl | N | C | N | 3.56b | ||
| 49 | C | -S-phenylpropyl | H | 5.87b | ||||||
| 50 | C | -S-phenyl | H | 4.04b | ||||||
| 51 | C | -S-2-methylbenzyl | H | 5.20b | ||||||
| 52 | C | -S-3-methylbenzyl | H | 4.92b | ||||||
| 53 | C | -S-4-methylbenzyl | H | 6.34b | ||||||
| 54 | C | -S-benzyl | NH2 | 5.28b | ||||||
| 55 | C | -S- (3-bromo-benzyl) | NH2 | 5.54b | ||||||
| 56 | C | -S- (4-bromo-benzyl) | NH2 | 6.09b | ||||||
| 57 | D | -S-isopropyl | NH2 | butyl | N | C | N | 3.92b | ||
| 58 | D | -S-methyl | NH2 | butyl | N | C | N | 3.66b | ||
| 59 | D | -S- (2-pyridylmethyl) | NH2 | butyl | N | C | N | 4.28b | ||
| 60 | C | -S- butyl | NH2 | 4.92b | ||||||
| 61 | C | -S- sec-butyl | NH2 | 4.20b | ||||||
| 62 | C | -S- (2-chloro-benzyl) | NH2 | 4.87b | ||||||
| 63 | C | -S- ethyl | NH2 | 3.79b | ||||||
| 64 | C | -S- (2-fluoro-benzyl) | NH2 | 5.32b | ||||||
| 65 | C | -S- (4-fluoro-benzyl) | NH2 | 5.94b | ||||||
| 66 | C | -S- (2-hydroxy-5-nitrobenzyl) | NH2 | 8.56b | ||||||
| 67 | C | -S- iodo | NH2 | 4.09b | ||||||
| 68 | D | -S-propyl | NH2 | isobutyl | N | C | N | 4.2b | ||
| 69 | C | -S- isobutyl | NH2 | 5.06b | ||||||
| 70 | D | -S-isopropyl | NH2 | propyl | N | C | N | 3.84b | ||
| 71 | C | -S- isopropyl | NH2 | 3.72b | ||||||
| 72 | D | -S- (2-pyridylmethyl) | NH2 | 2-methylbutyl | N | C | N | 4.38b | ||
| 73 | C | -S- (1-methyl-4-nitroimidazol-5-yl) | NH2 | 3.24b | ||||||
| 74 | C | -S- (6-methyl-2-pyridylmethyl) | NH2 | 1.94b | ||||||
| 75 | C | -S- methyl | NH2 | 3.69b | ||||||
| 76 | C | -S- 2-nitrobenzyl | NH2 | 5.34b | ||||||
| 77 | C | -S- 3-nitrobenzyl | NH2 | 6.75b | ||||||
| 78 | C | -S- phenethyl | NH2 | 4.79b | ||||||
| 79 | D | -S- (2-pyridylmethyl) | NH2 | propyl | N | C | N | 4.04b | ||
| 80 | C | -S- propyl | NH2 | 4.49b | ||||||
| 81 | C | -S- (2-pyridyl-methyl) | NH2 | 4.46b | ||||||
| 82 | C | -S- (3-pyridyl-methyl) | NH2 | 4.83b | ||||||
| 83 | C | -S- (2-acetophenone) | NH2 | 3.35b | ||||||
| 84 | C | -S- (4′-chloro-2-acetophenone) | NH2 | 4.84b | ||||||
| 85 | D | -NH- isopentyl | H | H | H | C | N | N | 3.63b | |
| 86 | D | -NH- phenethyl | H | H | H | C | N | N | 4.00b | |
| 87 | D | -NH2 | H | -β-D-ribofuranosyl | I | C | C | N | 3.64b | |
| 88 | D | -S- benzyl | H | -β-D-ribofuranosyl | H | C | C | N | 5.28b | |
| 89 | D | -S- methyl | H | -β-D-ribofuranosyl | Br | C | C | N | 3.94b | |
| 90 | D | -chloro | H | -β-D-ribofuranosyl | I | C | C | N | 4.11b | |
| 91 | D | -methoxy | H | -β-D-ribofuranosyl | H | C | C | N | 3.47b | |
| 92 | D | -piperidino | H | -β-D-ribofuranosyl | H | C | C | N | 3.71b | |
| 93 | D | -SH | H | -β-D-ribofuranosyl | H | C | C | N | 3.41b | |
