Fig. 2.
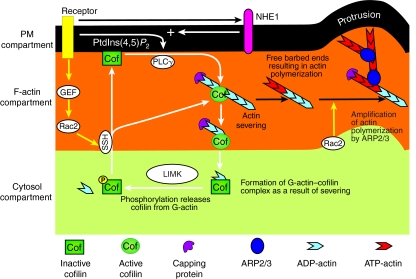
The common cofilin activity cycle in invasive tumor cells and inflammatory cells. Cofilin molecules cycle through three compartments in the cell: the cytosol, F-actin and the PM compartments. Cofilin is inactivated at the PM by binding to PtdIns(4,5)P2, and in the cytosol by either low pH levels or phosphorylation. Upon activation, cofilin translocates from the PM and cytosolic compartments to the F-actin compartment. The translocation of cofilin from the PM to the F-actin compartment is induced by a PLCγ-mediated decrease in PtdIns(4,5)P2 levels, which is amplified by an increase in pH (mediated by the Na+-H+ exchanger NHE1), which reduces the affinity of cofilin for PtdIns(4,5)P2, whereas the translocation from the cytosol to the PM compartment is induced by dephosphorylation of cofilin. At the F-actin compartment, cofilin binds to and severs actin filaments, which results in filaments with free barbed ends and the formation of cofilin–G-actin complexes. These complexes cannot bind to actin or the PM, and therefore diffuse to the cytosol compartment, where cofilin is phosphorylated and released from the cofilin–G-actin complex by LIMK. A new cycle is started when cofilin is dephosphorylated (by SSH, for example), which results in the re-entry of cofilin to the PM or F-actin compartment. The generation of actin free barbed ends by cofilin initiates the polymerization of new actin filaments to which the ARP2/3 complex prefers to bind, which amplifies cofilin-induced actin polymerization and results in the formation of cellular protrusions. `+' indicates pH increase.