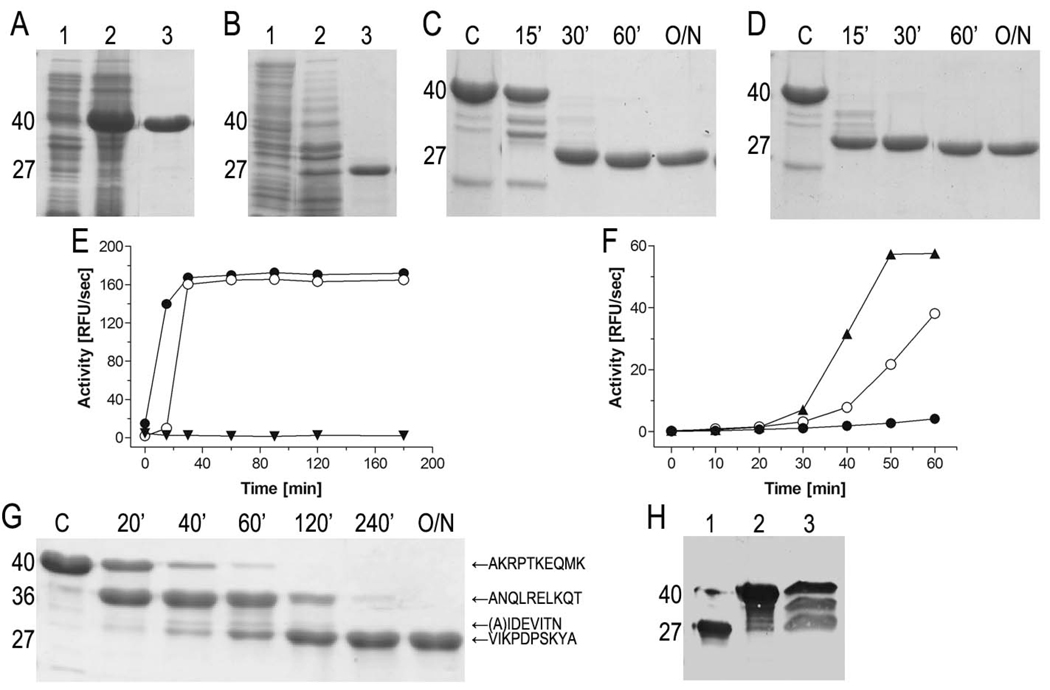
Figure 2. Expression and activity of InpA.
(A) Expression and purification of pro-cd-InpA C154A. Lanes 1 and 2, E. coli homogenate before and 3h after protein expression induction, respectively. Lane 3, recombinant protein after affinity chromatography purification. Molecular masses of the distinct protein species (40 and 27 kDa) are shown on the left. (B) Same for wt pro-cd-InpA. (C) Time-course analysis of autocatalytic processing and activation of wt pro-cd-InpA (final concentration 10µM) incubated with 1mM HgCl2. The reaction was initiated by adding EDTA (5mM final concentration) as a Hg2+-chelator, i.e. by releasing metal-mediated inhibition. Samples were withdrawn at the time intervals specified (O/N, overnight incubation; lane C, pro-cd-InpA alone). (D) Same as (C) but after addition of active InpA (10nM final concentration) to the reaction mixture. In this case, the reaction proceeded much faster. (E) A subset volume of the withdrawn aliquots from (C) and (D) was used to quantify the activity released from wt pro-cd-InpA in the absence (○) and presence (●) of catalytic amounts of wt cd-InpA. As a control, pro-cd-InpA spiked with InpA but without EDTA was incubated in parallel (▼). (F) Concentration dependent autoactivation of pro-cd-InpA. The reaction was initiated by releasing Hg2+-mediated inhibition in mixtures containing 0.04µM (●), 2µM (○) and 10µM (▲) zymogen. At indicated time points, 50µl (●), 10µl (○), and 2µl (▲) were withdrawn from each reaction mixture and directly assayed for activity. (G) SDS-PAGE of the digestion of pro-cd-InpA C154A by wt cd-InpA. The zymogen (final concentration of 10µM) was incubated with cd-InpA (0.1µM) for time intervals as specified (lane C, control pro-cd-InpA C154 incubated alone). N-terminal amino acid sequences of pro-cd-InpA derived fragments are indicated on the right. (H) Western blot analysis of culture supernatant of P. intermedia using InpA-specific rabbit antiserum (lane 3). Wt cd-InpA and pro-cd-InpA (C154A) were loaded on lane 1 and 2, respectively, for comparison.