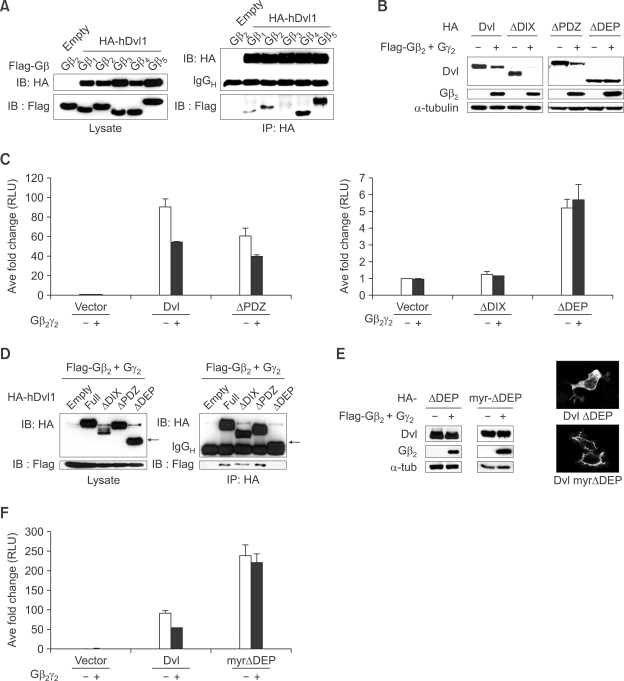
Figure 3.
DEP domain of Dishevelled (Dvl) is required for degradation of Dishevelled by Gβγ. (A) Dishevelled interacts with all isoforms of Gβ except for Gβ3. HA-tagged human Dv-1 was co-transfected with all isoforms of Gβ into HEK293T cells. Immunoprecipitation followed by western blotting was performed. (B) The level of DEP domain deleted Dishevelled was not reduced by Gβ2γ2. Various deletion constructs of Dishevelled were co-transfected with Gβ2γ2 into HEK293T cells and western blotting was performed. (C) The data from luciferase assay confirm the result obtained in (B). Luciferase assay was performed using superTOPFlash and pTK-renilla luciferase after co-transfection of various deletion constructs of Dishevelled and Gβ2γ2. (D) DEP domain of Dishevelled is required for interaction with Gβ2γ2. Various deletion constructs of Dishevelled with Gβ2γ2 were co-transfected into HEK293T cells and immunoprecipitation followed by western blotting was performed. Arrow indicates ΔDEP-Dishevelled. (E) Membrane localization of ΔDEP-Dishevelled via myristoylation/ palmitoylation signal is not sufficient for the down-regulation of Dishevelled by Gβ2γ2. ΔDEP-Dishevelled and myr-ΔDEP, which has a myristoylation/ palmitoylation signal sequence to the N-terminal of ΔDEP-Dishevelled, were transfected alone or with Gβ2γ2 into HEK293T cells. After fractionation, western blotting was performed using the membrane fraction (left panel). Indirect immunofluorescence analysis with anti-HA antibody shows myr-ΔDEP-Dishevelled is highly localized in plasma membrane (right panel). (F) Luciferase assay using superTOPFlash was conducted to confirm the result obtained in (E).