Abstract
During the latter stages of development in fleshy fruit, water flow through the xylem declines markedly and the requirements of transpiration and further expansion are fulfilled primarily by the phloem. We evaluated the hypothesis that cessation of water transport through the xylem results from disruption or occlusion of pedicel and berry xylem conduits (hydraulic isolation). Xylem hydraulic resistance (Rh) was measured in developing fruit of grape (Vitis vinifera ‘Chardonnay’) 20 to 100 d after anthesis (DAA) and compared with observations of xylem anatomy by light and cryo-scanning electron microscopy and expression of six plasma membrane intrinsic protein (PIP) aquaporin genes (VvPIP1;1, VvPIP1;2, VvPIP1;3, VvPIP2;1, VvPIP2;2, VvPIP2;3). There was a significant increase in whole berry Rh and receptacle Rh in the latter stages of ripening (80–100 DAA), which was associated with deposition of gels or solutes in many receptacle xylem conduits. Peaks in the expression of some aquaporin isoforms corresponded to lower whole berry Rh 60 to 80 DAA, and the increase in Rh beginning at 80 DAA correlated with decreases in the expression of the two most predominantly expressed PIP genes. Although significant, the increase in berry Rh was not great enough, and occurred too late in development, to explain the decline in xylem flow that occurs at 60 to 75 DAA. The evidence suggests that the fruit is not hydraulically isolated from the parent plant by xylem occlusion but, rather, is “hydraulically buffered” by water delivered via the phloem.
The development of grape (Vitis vinifera) berries is typical of many fleshy fruits, following a double sigmoid pattern of growth with three distinct phases: an initial phase of rapid cell division and expansion in green berries, a short transitory phase of very little growth, and a final phase in which growth is reinitiated and the fruit ripens. The transition to the ripening phase is accompanied by many physiological changes, such as the production of anthocyanins and fruit softening. In grape, these distinctive and highly visible physiological changes are collectively referred to as veraison. The rapid accumulation of sugars that is initiated in the berry mesocarp around the time of veraison is accompanied by a dramatic shift in the proportion of xylem and phloem transport (Lang and Thorpe, 1989; Greenspan et al., 1994, 1996). This same shift, albeit more gradual, occurs in many other fleshy fruits such as tomato (Solanum lycopersicum; Ho et al., 1987), apple (Malus domestica; Lang and Ryan, 1994; Drazeta et al., 2004), and kiwifruit (Actinidia deliciosa; Dichio et al., 2003) as well as in the flowers of tropical trees (Chapotin et al., 2003). The sudden reduction in xylem transport to the fruit is perceived as a mechanism to hydraulically isolate the fruit and buffer them from environmental stresses experienced by the parent plant.
Using mass balance techniques, Greenspan et al. (1994, 1996) reported major changes in the role of the xylem and phloem in water transport to the grape berry at veraison. During the first growth phase, the xylem provides the majority of water transport into the berry. In the final growth stage, the phloem provides more than 80% of the berry's water requirements and the contribution of the xylem becomes negligible. Berry water status also becomes apparently uncoupled from plant water status after veraison. Before veraison, diurnal contractions in berry diameter were strongly related to changes in plant (stem) water potential, while after veraison, diurnal contractions were greatly reduced and unrelated to changes in stem water potential (Matthews and Shackel, 2005). A similar lack of response was also observed for mesocarp cell turgor after veraison (Thomas et al., 2006). Thus, it is clear that some mechanism acts to decouple berry water relations from the water status of the parent plant.
Over the past two decades, a general consensus has developed that the berry xylem becomes physically disrupted after veraison, effectively blocking the xylem pathway and isolating the fruit essentially as a whole from the parent plant (During et al., 1987; Findlay et al., 1987; Lang and Ryan, 1994). Evidence for this has been provided by observations of dye uptake into the berry through the xylem. When the cut pedicel of a preveraison berry is submerged in dye, the dye is taken up into peripheral and axial xylem conduits of the entire berry (Findlay et al., 1987; Creasy et al., 1993; Rogiers et al., 2001). After veraison, dye uptake is limited to the base of the berry vasculature (brush). From this evidence, together with micrographs that appeared to show stretched and ruptured xylem conduits in postveraison berries, it was inferred that the lignified tracheids present at veraison were physically torn apart by the expansion of the berry that occurred postveraison.
Recent experimental work using a range of techniques suggests that the hypothesis of physical disruption may be oversimplified and that the berry xylem remains at least potentially functional after veraison (Bondada et al., 2005; Keller et al., 2006; Chatelet et al., 2008b). The results of Chatelet et al. (2008a, 2008b) demonstrate that the majority of xylem conduits in the berry remain intact after veraison and suggest that xylem development (growth of new conduits) continues well into the postveraison growth phase. Using both a modified pressure plate/membrane apparatus and a wicking technique, it was demonstrated that dye moved through the xylem of postveraison berries when a hydrostatic pressure or matric gradient was applied between the pedicel and the cut stylar surface (Bondada et al., 2005; Chatelet et al., 2008b). Keller et al. (2006) demonstrated this in reverse, showing that berry xylem was still capable of conducting a dye tracer back to the parent plant if the dye was introduced at the cut stylar end while the plant was transpiring. Thus, given a large enough pressure gradient, the xylem of postveraison berries retains the potential to transport water between the parent plant and the berry or vice versa. However, anatomical measurements and dye tracer studies can only be used to infer the degree to which fruit may become isolated from the parent plant. Knowledge of changes in hydraulic resistance (Rh) is required to determine whether xylem dysfunction is actually responsible for declining xylem flows reported with the progression of ripening. It is also important to differentiate between xylem flows and Rh, as these variables are sometimes confused in the literature; xylem flow rates can vary independently of Rh if water potential gradients along the pathway are altered.
Previous studies examining changes in Rh associated with the development of fleshy fruit generally indicate that Rh increases during ripening but show differences in the timing and location of the increase. Some fruits develop an abscission zone in the pedicel or receptacle that is associated with vascular constriction and high Rh (Mackenzie, 1988; Lee, 1989; Van Ieperen et al., 2003). However, although some table grapes are believed to develop an abscission zone, there is no evidence of an abscission zone in wine grapes (Pratt, 1971). Tyerman et al. (2004) reported a substantial increase in Rh of grape berries after veraison, although this increase in resistance did not occur in the pedicel or receptacle but mainly in the distal section of the berries. Similarly, Malone and Andrews (2001) evaluated Rh in developing tomato fruits and stems and found that Rh increased in the fruit, but not proximal to the calyx. In apple, Lang and Ryan (1994) observed an increase in Rh at 80 d after anthesis (DAA) and also reported an increasing proportion of samples in which the xylem was completely occluded with age. Although they described these data as pedicel Rh, their measurements actually included the fruit vascular pathway; therefore, it is difficult to determine if the increase in Rh was manifested in the fruit or the pedicel.
Increases in pedicel and receptacle Rh should be associated with changes in the dimensions or conductive state or xylem conduits. An increase in the Rh within the fruit may relate either to xylem dysfunction or to extravascular resistance beyond the xylem. Although they were not able to partition an increase in fruit Rh between the apoplast and symplast, Tyerman et al. (2004) suggested that the site of increased resistance may be the plasma membranes of vascular parenchyma cells separating xylem conduits and mesocarp cells rather than the xylem itself. A likely candidate driving hydraulic isolation at the cellular level is changes in plasma membrane Rh resulting from the differential expression and activity of aquaporins. Aquaporins are a family of transmembrane proteins considered to be largely responsible for the high permeability to water exhibited by plasma membranes. The regulation of Rh by aquaporins is now well documented in roots (Martre et al., 2001; McElrone et al., 2007; Vandeleur et al., 2009), and oxidative gating of aquaporins has been reported to reduce hydraulic conductivity by 90% in cells of the giant algae Chara (Henzler et al., 2004). The results of previous work suggest that aquaporins play an important role in the regulation of water movement during the development of flowers, seeds, and fruits (Maurel et al., 1995; Gao et al., 1999; Picaud et al., 2003; Shiota et al., 2006; Zhou et al., 2007). Changes in the expression of the plasma membrane intrinsic protein (PIP) PIP1 and PIP2 aquaporin gene families have been noted in ripening grapes (Picaud et al., 2003; Fei et al., 2004), although the effects of these changes on water transport (membrane conductivity) have not been documented. An increase of Rh between the mesocarp cells and the xylem within the fruit could provide a mechanism to restrict water movement between the parent plant and the berry if a large gradient in xylem tension existed between the two (Tyerman et al., 2004).
While the concept of hydraulic isolation is generally accepted as part of the physiology of fleshy fruit development, we note that no studies have demonstrated an increase in Rh that is coincident with the decline in xylem flow. Additionally, measured variation in the Rh of the fruit and pedicel has not been quantitatively related to the water requirements of the fruit, taking into account water potential gradients between the fruit and the parent plant. In this study, we examined changes in the Rh of the berry, receptacle, and pedicel of Chardonnay grape over the course of fruit development. These measurements were compared against observations of xylem anatomy and aquaporin gene expression in order to investigate the hypotheses that (1) occlusion and/or disruption of xylem conduits results in the hydraulic isolation of ripening grape berries, and (2) an increase in the Rh of the berry is associated with changes in the expression of aquaporin genes in the mesocarp.
RESULTS
Changes in Rh with Development
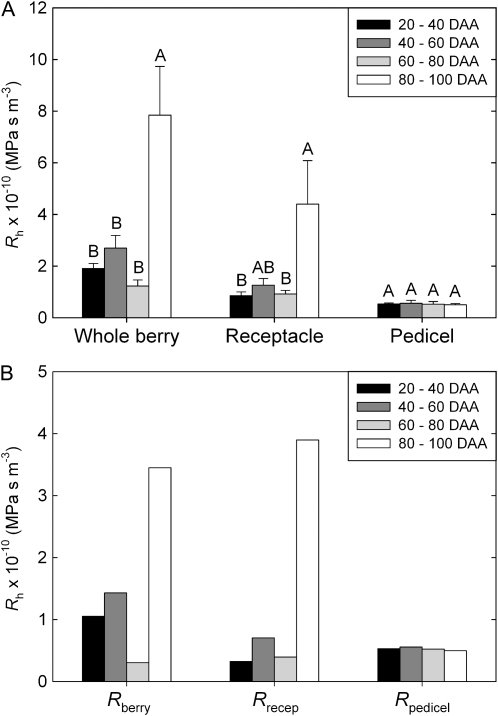
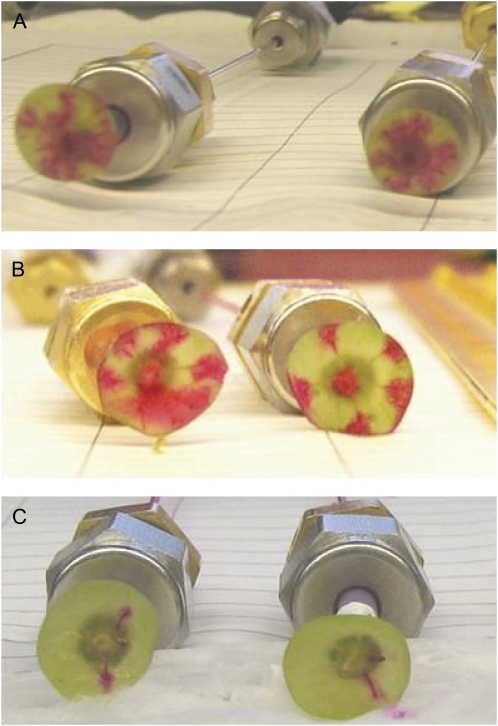
There was no significant change in Rh of the whole berry, receptacle, or pedicel until 80 DAA (Fig. 1A). During the late postveraison stage (80–100 DAA), there were significant increases (P < 0.05) in Rh of the whole berry and receptacle. The Rh of the pedicel did not change significantly across development. Dye tracers using acid fuchsin showed that the increase in whole berry and receptacle Rh at 80+ DAA was associated with a decrease in the number of vascular strands conducting at a delivery pressure of 0.1 MPa (Fig. 2C). In samples collected before veraison (Fig. 2A) and up to 75 DAA (Fig. 2B), the dye tracer moved through all peripheral and axial vascular strands when viewed in transverse section through the brush.
Figure 1.
Changes in hydraulic parameters of whole berries, receptacles, and pedicels over the course of development (early preveraison = 20–40 DAA; late preveraison = 40–60 DAA; veraison/early postveraison = 60–80 DAA; late postveraison = 80–100 DAA). A, Measured Rh at each cut section (MPa s−1 m−3). B, Rh calculated for each component of the berry by subtraction, with Rberry = Rh (whole berry) – Rh (receptacle) and Rrecep = Rh (receptacle) – Rh (pedicel). Values are means with error bars showing se. For each parameter, different letters indicate significant differences (P < 0.05) between means (Tukey's honestly significant difference).
Figure 2.
Perfusion of acid fuchsin into grape berries cut at the brush region at different stages of development. Images are shown for berries at 30 DAA with central and all peripheral vascular bundles conducting (A), at 75 DAA with central and all peripheral vascular bundles remaining conductive (B), and at 100 DAA with only a small number of bundles still conducting dye (C). [See online article for color version of this figure.]
Because resistances are additive in series, the resistance of each component (berry, receptacle, and pedicel) could be calculated by subtraction. This is necessary because the measured whole berry Rh includes the receptacle and pedicel resistances and the receptacle Rh includes the pedicle resistance. Rh of each section was calculated as Rberry = Rh (whole berry) – Rh (receptacle) and Rrecep = Rh (receptacle) – Rh (pedicel; Fig. 1B). This analysis showed that Rberry was lowest during the period just after veraison at 60 to 80 DAA. Both Rberry and Rrecep increased significantly in the late postveraison period at 80 to 100 DAA and contributed in roughly equal proportion to the increase in whole berry Rh at this stage. Changes in the Rrecep are presumed to relate primarily to the conductive state of xylem conduits, since conduits were cut open at both ends of the sample for these measurements.
Cryo-Scanning Electron Microscopy and Light Microscopy
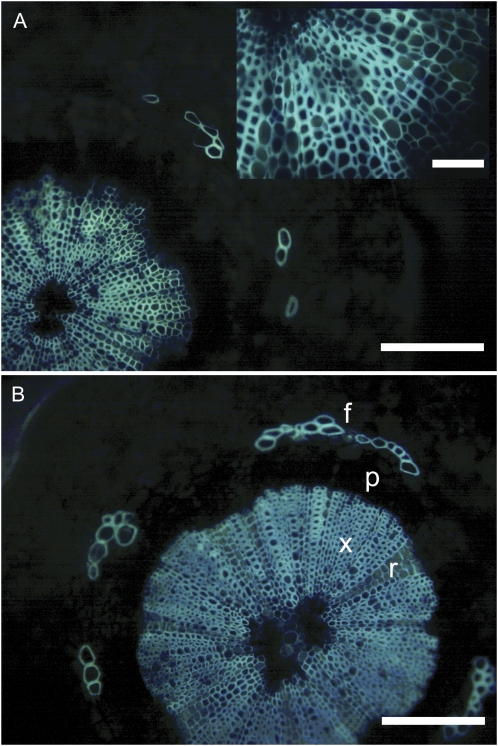
Light microscope images showed that the structure and organization of xylem tissue were similar between preveraison and postveraison berries (Fig. 3). The xylem tissue consists of rays with thin-walled cells and a more heavily lignified matrix of fibers and tracheary elements. The conducting elements were in the range of 5 to 30 μm in diameter and were often hard to discern from surrounding fibers and parenchyma based on light microscopy because of the overlap in cell size.
Figure 3.
Light micrographs showing transverse sections of pedicel tissue in grape berries stained with berberine-aniline blue. A, Preveraison pedicel (30 DAA), with inset showing a high-magnification view of xylem tissue. B, Postveraison pedicel (75 DAA) showing heavily lignified xylem (x), thinner walled ray cells (r), and fiber caps (f) positioned over phloem tissue (p). Bars = 100 μm or 50 μm for the inset. [See online article for color version of this figure.]
Cryo-scanning electron microscopy (cryoSEM) can be used to determine whether xylem conduits are functional (water filled) or embolized (gas filled) at the time of freezing (McCully et al., 2009). In addition, it provides information on the distribution and concentrations of solutes in the sample. Within cells of the frozen sample, sequestered solutes appear as a condensed reticulum of bright electron-emissive lines in the frozen water, which appears much darker in contrast. This patterning of solutes is an artifact of freezing caused by the exclusion of solutes by ice crystal growth. The density of the reticulum gives an indication of the solute concentration before freezing or the presence of gels (Crews et al., 2003; Ball et al., 2006). Living cells show a very dense patterning because of high cytoplasmic and vacuolar solute concentrations. Tracheary elements generally contain a much lower concentration of solutes and therefore appear darker than surrounding living cells (Canny, 1997).
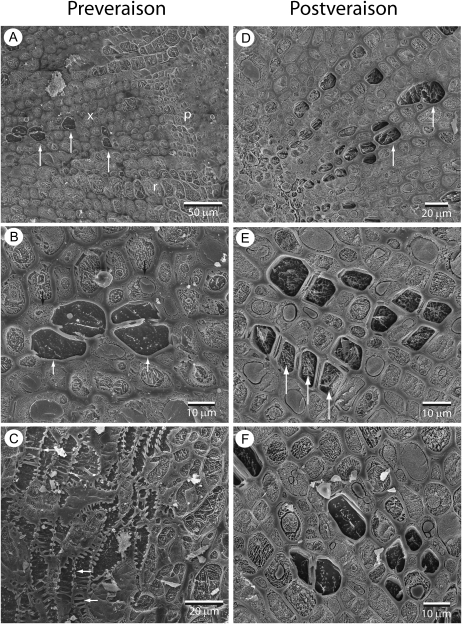
In receptacles of early preveraison berries (31 DAA), tracheary elements could be differentiated from other cell types of the xylem by the presence of bordered pits and because they contained dark ice, indicating a low concentration of solutes compared with surrounding living cells (Fig. 4, A and B). Tracheary elements could also be identified by the presence of bordered pits or annular secondary thickenings (Fig. 4C). No embolized or occluded tracheary elements were observed in preveraison samples. In the receptacles of late postveraison berries (91–133 DAA), many tracheary elements contained dense patterns of sequestered solutes (Fig. 4, D–F). There was considerable variation in the amount of sequestered material in tracheary element sap, with some elements almost completely packed (Fig. 4E); others appeared to have a similarly low solute concentration to tracheary elements in preveraison samples and were easily distinguishable from sounding cells by the darkness of the ice (Fig. 4F). Overall, 72% of tracheary elements seen in cross-sections of postveraison receptacles had a high density of sequestered material compared with tracheary elements in preveraison samples. No gas-filled conduits were detected in postveraison receptacles, indicating that xylem conduits were not embolized at the time of freezing. Thus, cryoSEM observations suggest that there was a substantial increase in the solute concentration of sap or deposition of gel material in xylem tracheary elements late in ripening, but they did not show evidence of tyloses or embolism in the vascular system.
Figure 4.
CryoSEM images showing pedicel and receptacle tissue for preveraison (31 DAA; A–C) and postveraison (93–100 DAA; D–F) grape berries. A, Transverse section through receptacle showing xylem (x), phloem (p), and ray (r). Conducting tracheary elements (arrows) appear dark in comparison with surrounding living cells because of low solute content in the ice. B, Transverse section showing closer detail of conducting elements (white arrows) surrounded by living cells in which organelles can be seen (black arrows). C, Transverse section showing lateral branching of a vascular bundle. Tracheary elements can be seen connected through bordered pits and annular secondary thickening (arrows). D, Transverse section through receptacle showing tracheary elements containing high amounts of solute relative to preveraison samples. E, Closer detail showing many tracheary elements containing white lacy patterning indicating high concentrations of solutes (arrows). F, Transverse section showing some postveraison tracheary elements that appear similar to preveraison samples with lower solute concentrations.
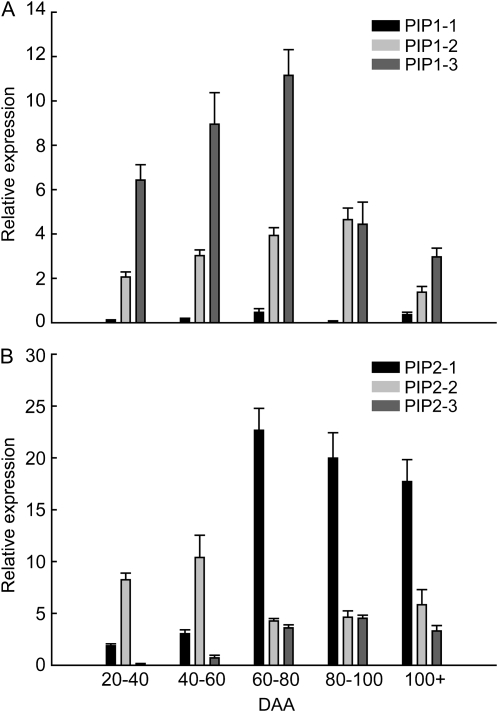
Aquaporin Gene Expression
Variation in berry Rh could also be mediated by changes in the expression and activity of aquaporins. Therefore, the mRNA expression of six aquaporin genes in grape berries was assessed with quantitative PCR over the course of development. Although each of the six PIP1 and PIP2 genes showed distinct patterns of expression during the development of the berry, overall expression of PIP genes was higher after veraison, particularly at 60 to 80 DAA (Fig. 5). The degree to which different isoforms contributed to total aquaporin gene expression changed between preveraison and postveraison. Prior to veraison, VvPIP1;3 and VvPIP2;2 were the most abundantly expressed aquaporin genes for PIP1 and PIP2 families, respectively. VvPIP2;2 was the only aquaporin gene that was more highly expressed before veraison than after veraison. At 60 to 80 DAA, expression was up-regulated in all isoforms except VvPIP2;2. In the most abundantly expressed isoform, VvPIP2;1, expression increased almost 10-fold after veraison. The expression of most aquaporin genes decreased 60 to 100 DAA, but expression of many PIP genes remained relatively high even after 100 DAA.
Figure 5.
Aquaporin in grape berry flesh over the course of development. Isoforms are separated into the PIP1 (A) and PIP2 (B) gene families. Values are means with bars showing se across all biological and technical replicates.
Flow Model
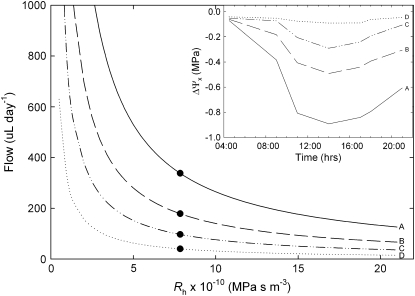
A flow model was developed to examine whether increasing berry and receptacle Rh could account for hydraulic isolation of the berry after veraison. The model predicted that significant backflow was still possible from the berry into the parent plant even with the increase in Rh observed in the latter stages of ripening (80–100 DAA; Fig. 6). For a given diurnal time course of xylem water potentials (Fig. 6, inset), the model shows that increasing Rh results in decreasing backflow from the berry to the parent plant over the course of 1 d. If the berry xylem water potential is high and stable at 0.02 MPa (Tyerman et al., 2004), a backflow of 338 μL d−1 is expected based on the whole berry Rh measured 80 to 100 DAA (Fig. 6, scenario A). As the xylem water potential gradient between the fruit and plant deceases, the predicted backflow for the measured Rh decreases to values ranging from 40 to 180 μL d−1 (Fig. 6, scenarios B–D). It is recognized that other factors, particularly the exchange of water between apoplastic and symplastic components, would influence the direction and magnitude of flow. However, in this case, we are concerned simply with demonstrating the potential for the measured changes in xylem Rh to cause the hydraulic isolation of the fruit.
Figure 6.
The relationship between grape berry Rh and backflow from the fruit into the parent plant. Flow rates were calculated using Rh and xylem water potential gradients (ΔΨx) between the fruit and the stem xylem. Four scenarios of diurnal time course of ΔΨx are shown (A–D) in the inset, with diurnal time courses in stem water potential based on data for irrigated postveraison vines from Greenspan et al. (1996). The black circles show mean whole berry Rh measured at 80 to 100 DAA.
DISCUSSION
Our results show that although a decrease in expression of some aquaporin genes and an increase in berry and receptacle Rh are identifiable in the data, these impediments to water transport are relatively small in magnitude and occur too late in the development of the fruit to explain the switch from xylem to phloem transport observed after veraison. The expression of VvPIP2;2 decreased by 50% after 60 DAA; however, the expression of VvPIP2;1 increased 10-fold at the same time. There was no significant change in Rh of whole berries until 80 DAA, when a 4-fold increase occurred. The Rh of the receptacle exhibited a similarly late increase of roughly equal magnitude (Fig. 1). The timing of the changes in berry and receptacle Rh was such that the increase in Rh occurred after the ripening of the fruit had progressed considerably, with Brix above 15 degrees and the second growth phase essentially complete (Chatelet et al., 2008b; Wada et al., 2008). In contrast to becoming isolated, the hydraulic conductance remaining at 80 DAA was sufficient to support a high rate of water recycling to the parent plant during the day. Previous measurements on Shiraz and Chardonnay grape berries also indicated that there was not a rapid increase in Rh at veraison, with Rh of the fruit only increasing substantially at 75 to 110 DAA (Tyerman et al., 2004). Studies of apple show similar results, with a consistent increase in Rh seen only after 90 DAA (Lang and Ryan, 1994).
In some previous studies, Rh increases were localized to the fruit itself and were reported not to occur in the receptacle or pedicel (Malone and Andrews, 2001; Tyerman et al., 2004). Our study provides clear evidence for a late ripening (80–100 DAA) increase in both the receptacle and fruit Rh for grape berries. Candidates for an increase in receptacle Rh include the formation of tyloses, gels, or embolism in xylem conduits. Some previous work has shown that tyloses form in the pedicel and receptacle of xylem conduits after veraison in Thompson Seedless grapes (Kasimatis, 1957). However, cryoSEM observations offered no evidence of tyloses or embolisms forming in the conduits of the receptacle after veraison but showed many conduits containing a high concentration of sequestered material. Although the exact nature of the material observed with cryoSEM was not determined, it is expected that a high concentration of solutes would substantially increase the Rh in affected conduits via their effects on viscosity. One possibility is that the solutes are polysaccharide gels, as the density of their appearance resembles that of previously published cryoSEM images of polysaccharide gels deposited in xylem vessels (Crews et al., 2003). Alternatively, the xylem solutes may be sugars that have been recycled from the berry phloem or the berry apoplast, given the large postveraison increase in apoplastic solute concentration (Wada et al., 2008).
As with previous work, we observed a large increase in the Rh of the fruit itself, excluding any increases in resistance that may occur in the receptacle (Tyerman et al., 2004). Unlike the pedicel and receptacle, increases in Rh measured for the berry may result not only from increases in xylem Rh but also from the resistance to water movement between the xylem and symplastic components of the berry (i.e. increases in the resistance of plasma membrane of mesocarp parenchyma cells surrounding the berry xylem). Therefore, the movement of water into and out of the berry may be influenced by the expression and activity of aquaporins in the berry mesocarp as well as by structural changes in xylem vessels.
Aquaporin Gene Expression and Changes in Rh
We observed significant differences in the expression profiles of aquaporin genes across development, with individual isoforms each having a specific pattern of expression. Generally, peaks in the expression of most isoforms corresponded to low whole berry Rh at 60 to 80 DAA. Specifically, the most predominantly expressed PIP1 and PIP2 isoforms, VvPIP1;3 and VvPIP2;1, show peaks of expression during this period. Expression studies by Fouquet et al. (2008) demonstrated similar patterns of expression in Cabernet Sauvignon grape for most isoforms; VvPIP1;3 and VvPIP2;1 were the most predominantly expressed isoforms, with peaks of expression at 70 DAA. One notable difference is that VvPIP1;2 had a peak expression at 20 DAA not detected in our study. Given that 20 DAA lies at an extreme of our sampling regimen, it is probable that this developmental stage was not represented in our study. In some cases, there are differences in the magnitude of changes in expression but not the pattern of these changes. For example, both studies found that VvPIP2;1 expression increased around 70 DAA, but Fouquet et al. (2008) found an approximate 0.5-fold increase compared with the much greater 4-fold increase reported here.
Past work has shown that expression of PIP2 isoforms results in greater membrane water permeability in Xenopus oocytes compared with PIP1s (Yamada et al., 1995; Chaumont et al., 2000), while PIP1s are often nonfunctional with regard to water movement and may be associated with the transport of solutes (Dordas et al., 2000). However, coexpression of particular PIP1 and PIP2 genes in Xenopus oocytes has been observed to increase membrane water permeability above levels measured with the expression of those genes alone (Fetter et al., 2004). In this work, Fetter et al. (2004) identified four PIP1 E-loop residues critical for this interaction, and the VvPIP1s in this study share 100% similarity across these residues (Fig. 7). Furthermore, interactive effects were not observed in Phaseolus vulgaris, where these residues were not conserved (Zhou et al., 2007).
Figure 7.
E-loop region amino acid sequence alignment of the grapevine PIPs (VvPIPs). Shaded residues in PIP1s represent those that Fetter et al. (2004) found to be critical for the heteromerization between maize (Zea mays) ZmPIP1-2 and ZmPIP2-5, leading to increases in membrane water permeability.
Recent work with grapevine by Vandeleur et al. (2009) showed the same interaction between VvPIP2;2 and VvPIP1;1. VvPIP1;1 had no effect on membrane water permeability when expressed in Xenopus oocytes, whereas VvPIP2;2 doubled membrane water permeability when expressed in the same system. As predicted by the conservation of those E-loop residues responsible for the synergistic interaction discussed above, coexpression of VvPIP1;1 and VvPIP2;2 resulted in a 6-fold increase in membrane water permeability. Thus, the coincidence of high levels of expression of VvPIP1s and VvPIP2s at 60 to 80 DAA is expected to result in additive increases in membrane hydraulic conductivity.
The substantial increase of Rh beginning at 80 DAA correlates with decreases in the expression of the predominantly expressed PIPs; however, numerous other isoforms show substantial levels of expression late into berry development. This was similar to the results of both Picaud et al. (2003) and Fouquet et al. (2008), who found that expression of many isoforms persisted at high levels until harvest. This suggests that transcriptional control of aquaporin expression may only partially contribute to the variation in Rberry observed across berry development. In addition, the influence of aquaporins on Rberry will depend on protein localization and posttranscriptional control. Changes in aquaporin expression and/or activity at specific locales in the pathway (i.e. parenchyma cells surrounding xylem vessels) would be expected to have a much higher impact on Rh. Therefore, in this and like studies, significant changes in aquaporin expression in these underrepresented tissues could be masked by changes taking place in tissues having minimal impact on Rh in general. Using in situ hybridization, Fouquet et al. (2008) indeed observed that in preveraison berries (20 DAA) expression of VvPIP2;1 and the tonoplast aquaporin VvTIP2-1 were localized to specific tissues, the seed and xylem parenchyma, respectively.
Tyerman et al. (2004) proposed that aquaporins may be differentially expressed or activated between xylem parenchyma preveraison, allowing for rapid uptake of water through the xylem, and phloem parenchyma postveriason, increasing the resistance of water movement between xylem and the mesocarp symplasm while facilitating the movement of water through the phloem. Similarly, Fouquet et al. (2008) speculated that decreases in the expression of VvTIP2-1 at veraison could increase the resistance of water movement between the xylem and berry tissue, based on their results localizing VvTIP2-1 expression to xylem parenchyma very early in berry development (20 DAA). Unfortunately, no localization data are available for aquaporin gene expression in postveraison grape berries, presumably due to problems associated with the fixation of postveraison berry tissue. Resolving the impact of aquaporin expression and activity will require more precise protein localization and assays of membrane permeability measured at different stages of development.
Alternatively, aquaporin expression may not significantly impact Rberry but may contribute to the ripening process in other ways. Recent studies have demonstrated that there is a large increase in hydrogen peroxide production postveraison (Pilati et al., 2007), and aquaporins have been shown to facilitate hydrogen peroxide transport across membranes (Bienert et al., 2007). In addition, the ripening process in general is associated with large increases in sugar transport and accumulation, changes in cell wall metabolism, and changes in turgor, all of which are certainly impacted by aquaporins and their modulation of membrane water permeability.
Impact of Changes in Rh and Aquaporin Expression on Xylem Function
The question remains how the postveraison increase in Rh relates to the concept of hydraulic isolation in ripening fruit. Multiple lines of evidence suggest that xylem transport of water into the berry becomes negligible after veraison, although there is some question over when exactly the decline in xylem transport commences and how rapid the decline is. While dye uptake and mass balance experiments suggest that flow through the xylem has virtually ceased at 65 to 75 DAA (Greenspan et al., 1994; Keller et al., 2006), the increase in Rh observed for receptacles and berry tissue in this study took place mainly at the late postveraison stage (80–100+ DAA). In fact, our results show that Rberry was lowest during the period directly after veraison, when xylem flows are seen to decline.
Our flow model indicates that the magnitude of increase in resistance alone would not be enough to prevent significant water loss from the berry given previously measured water potential gradients between postveraison grape berries and the parent plant (Fig. 6). Using a modified root pressure probe, Tyerman et al. (2004) showed that “pedicel equilibrium pressure” increased from negative values (indicating tension in the berry xylem) to close to zero after veraison. If the pedicel equilibrium pressure is an accurate reflection of the in situ tension of the berry xylem, then a substantial gradient in xylem water potential must exist between the berry and the stem xylem after veraison. The xylem backflow of 338 μL d−1 calculated from whole berry Rh values measured in our study represents a flow equal to roughly one-third of the total volume of water in the berry every day. Therefore, the large magnitude of predicted backflows could not occur unless the phloem was delivering an excess of water relative to the requirements of berry growth and transpiration. Alternatively, this could also be viewed as the volume of water that would be required to maintain berry xylem water potential close to zero and buffer the fruit from water stress experienced by the parent plant.
After veraison, phloem unloading in the berry switches from symplastic pathways to apoplastic pathways (Zhang et al., 2006) and solute concentrations in the apoplast increase to that of mesocarp cell symplastic solute concentration (Wada et al., 2008). Zhang et al. (2006) reports that phloem sap concentration in the pedicel remains at a consistently low value of around 50 mmol L−1 throughout berry development. If the phloem sap concentration remains low during ripening, then the sharp increase in rates of sugar accumulation around veraison must be the result of an increase in the volume of phloem sap entering the berry. When unloaded into the apoplast, this large volume of phloem water could act to reduce xylem tension in the berry and create a gradient favoring recycling of water back to the parent plant. This is further supported by the observations of Keller et al. (2006), which show that dye introduced at the cut stylar end of a postveraison berry is drawn back into the parent plant. If buffering of berry water status is maintained by phloemic influx of water, then it is also interesting to consider what might occur when the berry reaches maximum sugar concentration. Given that water influx through the phloem is tied to import of sugars, it is logical that phloem flow must decrease as the rate of sugar accumulation decreases in the fruit at around 100 DAA. If a xylem connection with relatively low Rh remains at this point, then one would expect tension to develop in the berry xylem and/or the loss of mass from the fruit due to backflow into the plant. However, the recent results of Tilbrook and Tyerman (2009) suggest that in some circumstances, Rh out of the berry is high enough to prevent significant backflow to the parent plant during the late ripening stage.
Overall, the evidence suggests that grape berries are not hydraulically isolated by increased xylem Rh during ripening, and we must draw a distinction between “isolation” and hydraulic “buffering,” which is achieved by some other mechanism. The delivery of excess phloem water and the accumulation of solutes in the apoplast could explain (1) the postveraison insensitivity of the berry to plant water deficits (Greenspan et al., 1994) and (2) the loss of turgor in berry mesocarp cells and softening of fruit that occurs during ripening (Thomas et al., 2006), without the need to invoke a physical loss of xylem continuity or increase in Rh. Therefore, the observed patterns of xylem and phloem flows over the course of fruit development may depend more on source/sink behavior than on simple hydraulic isolation. If this is the case, proper assessment of volume flow through the phloem after veraison and the extent of water recycling via the xylem will be essential to clarify mechanisms of water and sugar balance in ripening grapes and other fleshy fruit.
MATERIALS AND METHODS
Plant Material
Fruit were obtained from own-rooted grapevine (Vitis vinifera ‘Chardonnay’) grown in a greenhouse at the University of California, Davis. Vines were grown in 4-L pots filled with one-third peat, one-third sand, and one-third redwood compost, with 2.4 kg m−2 dolomite lime in a greenhouse. The vines were pruned to two shoots, and the shoots were vertically trained to approximately 1.5 m. Pots were drip irrigated four times a day for 4 min (2 L d−1) with dilute nutrient solution (90 μL L−1 calcium, 24 μL L−1 magnesium, 124 μL L−1 potassium, 6 μL L−1 nitrogen as NH4, 96 μL L−1 nitrogen as NO3, 26 μL L−1 phosphate, 16 μL L−1 sulfate, 1.6 μL L−1 iron, 0.27 μL L−1 manganese, 0.16 μL L−1 copper, 0.12 μL L−1 zinc, 0.26 μL L−1 boron, and 0.016 μL L−1 molybdenum at pH 5.5–6.0). Clusters were tagged at anthesis, and fruit was collected from vines between December 2006 and May 2007.
Veraison commenced 60 DAA for greenhouse-grown Chardonnay berries (Chatelet et al., 2008a; Wada et al., 2008). Thus, data collected before 60 DAA are for green berries and data collected from 60 to 100 DAA are for ripening berries.
Berry Rh
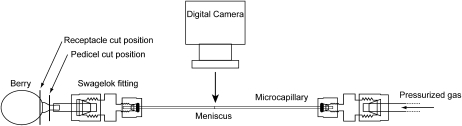
Rh (MPa s−1 m−3) was measured on berries harvested from the greenhouse from 30 to 100 DAA using a berry flow meter (Fig. 8). Clusters were collected from the vines between 8:30 and 9:00 in the morning, sealed in plastic bags, and returned to the laboratory within 15 min. Individual berries were cut from the cluster with a fresh razor blade under filtered, deionized water and sealed into Teflon tubing using cyanoacrylic glue. The tubing was then back filled with one of three perfusing fluids: deionized water, an aqueous solution of 0.01% acid fuchsin, or 10 mm KCl. These solutions were selected to determine if Rh was influenced by hydrogel behavior and a component of the berry vasculature (Zwieniecki et al., 2001). However, no significant difference was observed between these three perfusing fluids, and measurements were pooled for all perfusates. Rh was measured at three positions along the berry hydraulic pathway: the whole berry, the receptacle, and the pedicel. Cuts were made with a fresh razor blade at each position prior to beginning the Rh measurement (Fig. 8). Separate berries were used for each measurement because conductance of samples was observed to decline over time.
Figure 8.
Experimental apparatus used to measure Rh of grape berries, receptacles, and pedicels. Pedicels were glued into Teflon tubing and connected to a water-filled microcapillary. A gas pressure of 0.1 MPa was applied to the capillary, and the rate of water movement into the berry was measured by tracking the movement of the meniscus over time. The positions of cuts made to measure receptacle and pedicel Rh are shown.
Samples were connected to a glass microcapillary (i.d. = 0.5 mm) using a Swagelok reducing union. The other end of the microcapillary was connected to a pressurized gas source, and a gas pressure of 0.1 MPa was applied to the sample. The movement of the meniscus was tracked in the microcapillary by a digital camera mounted horizontally over the bench, which collected images every 60 to 120 s. The volume flow rate (m3 s−1) was calculated from the distance traveled along the capillary between each time point. Rh was calculated as Rh = ΔP/F, where ΔP is the pressure applied to the sample in MPa. Rh was calculated from the flow rate over a 120-s period beginning 400 to 600 s after the start of each measurement run. For whole berry measurements, uptake of water was monitored in one sample without the applied gas pressure. The rate of uptake was subtracted from the flow rate in other whole berry measurements for that cluster. The magnitude of this uptake was much greater before than after veraison. After the Rh measurement was complete, the weight, diameter, and degrees Brix of each berry were determined. For sampling, berry development was separated into four time periods: 20 to 40 DAA, 40 to 60 DAA, 60 to 80 DAA, and 80 to 100 DAA. For each time period, two to three clusters were collected and four measurements of Rh were made for each position on each cluster.
CryoSEM and Light Microscopy
For light microscopy, berries were hand sectioned and immediately stained according to Brundrett et al. (1988). Hand sections were stained with 0.1% berberine hemisulfate (Sigma-Aldrich), rinsed with distilled water, stained with 0.5% aniline blue (Sigma-Aldrich), rinsed again with distilled water, and then transferred to 0.1% FeCl3 in 50% glycerine. Sections were then mounted and visualized under UV light with an Olympus Vanox-AHBT (Olympus America) compound light microscope linked to a Pixera 600ES digital camera.
For cryoSEM, sample berries from the same cluster used in Rh measurements were frozen in liquid nitrogen at the time of collection. Samples were transferred under liquid nitrogen to the Center for Nanoscale Studies at Harvard University. Samples were mounted on aluminum stubs with TissueTek (Bayer) and planed with a glass knife at –140°C on an ultramicrotome with a cryobath attachment (Leica). After planing, mounted samples were transferred under liquid nitrogen to a SEM apparatus (FEI) equipped with a cold stage (Gatan). Samples were etched at –90°C for 5 to 10 min before being withdrawn to the prep chamber and sputter coated with gold. Samples were observed at –160°C in the SEM device, and digital images were taken for analysis.
Aquaporin Genes and Primer Design
Primer pairs were designed from submitted grape aquaporin cDNA sequences retrieved from National Center for Biotechnology Information GenBank. There is some confusion in the database regarding the gene designations arising from different groups submitting redundant gene sequences. Therefore, all cDNA sequences were aligned and compared. All primer pairs were designed to the heterogeneous 3′ untranslated region. The primer pairs were as follows: PIP1-1for (5′-GAGTGGTGCTGGGCGTTGATC-3′) and PIP1-1rev (5′-GTGGAATGCTACAGACATTAC-3′); PIP1-2for (5′-TCCTCCATTTTCTGTTTCTTC-3′) and PIP1-2rev (5′-ATTGTAATAGAAGCAGCCCAG-3′); PIP1-3for (5′-CCATCGCCTTTCTCTGTTGTG-3′) and PIP1-3rev (5′-AGAATACTCAATAATTTACAC-3′); PIP2-1for (5′-CCATTTTGATACCTTCTTCC-3′) and PIP2-1rev (5′-TATCTACAATTTCATGCCCTC-3′); PIP2-2for (5′-AACTAAAAACCCACAACACCC-3′) and PIP2-2rev (5′-CATCATCATAATCATCTCTGG-3′); and PIP2-3for (5′-CATTTCAATCCACATGGTCCG-3′) and PIP2-3rev (5′-CCACAAATTCGTCACACATCC-3′).
Aquaporin Gene Expression
Sample berries from the same cluster used in Rh measurements were frozen in liquid nitrogen at the time of collection. Samples were transferred to a −80°C freezer for storage. Total RNA was extracted, treated with DNase, and reverse transcribed following the methods described by Castellarin et al. (2007). Quantitative real-time PCR was carried out in an ABI PRISM 7700 sequence detector (Applied Biosystems). Each reaction (20 μL) contained 1 μm of each primer, 5 μL of 1:400 or 1:4,000 diluted cDNA, and 10 μL of Power SYBR Green Master Mix (Applied Biosystems). Thermal cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 56°C for 30 s, and 60°C for 60 s. Both cDNA dilutions were run in duplicate. Gene transcripts were normalized to VvUbiquitin1 (TC32075; The Institute for Genomic Research database) by comparing the cycle threshold of the target gene with that of VvUbiquitin1 (Bogs et al., 2005). Gene expression was expressed as mean and se calculated over all biological replicates.
Flow Model
In order to determine whether changes in Rh of the berry, receptacle, and pedicel could be responsible for hydraulic isolation, potential flow rates of water between postveraison grape berries and the parent plant were modeled. Flow rates through the xylem (F; μL h−1) were calculated as F = Rh ΔΨx, where ΔΨx is the xylem water potential gradient between the berry xylem and the stem xylem of the parent plant (Ψberry − Ψstem). Xylem water potential gradients were based on diurnal time courses (4:30 am–9:00 pm) of stem water potential for irrigated postveraison vines (−0.2 to −1.0 MPa; Greenspan et al., 1996). Four different scenarios (A–D) of ΔΨx were generated based on how closely xylem Ψberry tracked Ψstem over the course of the day. The greatest difference in Ψberry and Ψstem (scenario A) was based on previous measurements, which show a hydrostatic pressure close to zero (−0.01 to –0.02 MPa) in the xylem of late postveraison grape berries (Tyerman et al., 2004, B. Choat, unpublished data). Flow rates were calculated for a range of whole berry Rh at seven time points over the diurnal time course. Daily totals of water flow (μL d−1) were calculated by integrating the area under the diurnal curve. The whole berry Rh values for late postveraison (80–100 DAA) were included for each plot of potential flow.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers (protein, nucleotide): PIP1-1 ABN14347, EF364432; PIP1-2 ABN14348, EF364433; PIP1-3 ABN14349, EF364434; PIP2-1 (originally named PIP2-4) ABN14353, EF364438; PIP2-2 ABN14351, EF364436; and PIP2-3 ABN14352, EF364437.
Acknowledgments
We thank N. Michele Holbrook and Richard Schalek for assistance with cryoSEM observations, Simone Castellarin for help in developing aquaporin primers, and David Chatelet for assistance with fixation and sectioning of berry tissue. William Drayton is thanked for assistance with hydraulic measurements. Grapevines were donated by Cal Western Nurseries.
This work was supported by the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant no. 2005–34442–15841).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Brendan Choat (brendan.choat@anu.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Ball MC, Canny MJ, Huang CX, Egerton JJG, Wolfe J (2006) Freeze/thaw-induced embolism depends on nadir temperature: the heterogeneous hydration hypothesis. Plant Cell Environ 29 729–745 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Moller ALB, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282 1183–1192 [DOI] [PubMed] [Google Scholar]
- Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP (2005) Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol 139 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA (2005) Functional xylem in the post-veraison grape berry. J Exp Bot 56 2949–2957 [DOI] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146 133–142 [Google Scholar]
- Canny MJ (1997) Vessel contents during transpiration: embolisms and refilling. Am J Bot 84 1223–1230 [PubMed] [Google Scholar]
- Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA (2007) Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227 101–112 [DOI] [PubMed] [Google Scholar]
- Chapotin SM, Holbrook NM, Morse SR, Gutierrez MV (2003) Water relations of tropical dry forest flowers: pathways for water entry and the role of extracellular polysaccharides. Plant Cell Environ 26 623–630 [Google Scholar]
- Chatelet DS, Rost TL, Matthews MA, Shackel KA (2008. a) The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. J Exp Bot 59 1997–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Shackel KA, Matthews MA (2008. b) The peripheral xylem of grapevine (Vitis vinifera). 1. Structural integrity in post-veraison berries. J Exp Bot 59 1987–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy GL, Price SF, Lombard PB (1993) Evidence for xylem discontinuity in Pinot Noir and Merlot grapes: dye uptake and mineral composition during berry maturation. Am J Enol Vitic 44 187–192 [Google Scholar]
- Crews LJ, McCully ME, Canny MJ (2003) Mucilage production by wounded xylem tissue of maize roots: time course and stimulus. Funct Plant Biol 30 755–766 [DOI] [PubMed] [Google Scholar]
- Dichio B, Remorini D, Lang S (2003) Developmental changes in xylem functionality in kiwifruit: implications for fruit calcium accumulation. Acta Hortic 610: 191–195
- Dordas C, Chrispeels MJ, Brown PH (2000) Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124 1349–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazeta L, Lang A, Hall AJ, Volz RK, Jameson PE (2004) Causes and effects of changes in xylem functionality in apple fruit. Ann Bot (Lond) 93 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- During H, Lang A, Oggionni F (1987) Patterns of water flow in fiesling berries in relation to developmental changes in their xylem morphology. Vitis 26 123–131 [Google Scholar]
- Fei ZJ, Tang X, Alba RM, White JA, Ronning CM, Martin GB, Tanksley SD, Giovannoni JJ (2004) Comprehensive EST analysis of tomato and comparative genomics of fruit ripening. Plant J 40 47–59 [DOI] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay N, Oliver KJ, Nii N, Coombe BG (1987) Solute accumulation by grape pericarp cells. 4. Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries. J Exp Bot 38 668–679 [Google Scholar]
- Fouquet R, Leon C, Ollat N, Barrieu F (2008) Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep 27 1541–1550 [DOI] [PubMed] [Google Scholar]
- Gao YP, Young L, Bonham-Smith P, Gusta LV (1999) Characterization and expression of plasma and tonoplast membrane aquaporins in primed seed of Brassica napus during germination under stress conditions. Plant Mol Biol 40 635–644 [DOI] [PubMed] [Google Scholar]
- Greenspan MD, Schultz HR, Matthews MA (1996) Field evaluation of water transport in grape berries during water deficits. Physiol Plant 97 55–62 [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA (1994) Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant Cell Environ 17 811–820 [Google Scholar]
- Henzler T, Ye Q, Steudle E (2004) Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ 27 1184–1195 [DOI] [PubMed] [Google Scholar]
- Ho LC, Grange RI, Picken AJ (1987) An analysis of the accumulation of water and dry-matter in tomato fruit. Plant Cell Environ 10 157–162 [Google Scholar]
- Kasimatis AN (1957) Some factors influencing the development of water berries in Thompson Seedless grapes grown for table use. PhD thesis. University of California, Davis
- Keller M, Smith JP, Bondada BR (2006) Ripening grape berries remain hydraulically connected to the shoot. J Exp Bot 57 2577–2587 [DOI] [PubMed] [Google Scholar]
- Lang A, Ryan KG (1994) Vascular development and sap flow in apple pedicels. Ann Bot (Lond) 74 381–388 [Google Scholar]
- Lang A, Thorpe MR (1989) Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes principle. J Exp Bot 40 1069–1078 [Google Scholar]
- Lee DR (1989) Vasculature of the abscission zone of tomato fruit: implications for transport. Can J Bot 67 1898–1902 [Google Scholar]
- Mackenzie KAD (1988) The anatomy of fruit abscission in loganberries. Ann Bot (Lond) 62 249–263 [Google Scholar]
- Malone M, Andrews J (2001) The distribution of xylem hydraulic resistance in the fruiting truss of tomato. Plant Cell Environ 24 565–570 [Google Scholar]
- Martre P, North GB, Nobel PS (2001) Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiol 126 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MA, Shackel KA (2005) Growth and water transport in fleshy fruit. In NM Holbrook, MA Zwieniecki, eds, Vascular Transport in Plants. Elsevier Academic Press, Burlington, VT, pp 181–197
- Maurel C, Kado RT, Guern J, Chrispeels MJ (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin alpha-TIP. EMBO J 14 3028–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME, Canny MJ, Huang CX (2009) Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology: morphological and anatomical applications. Funct Plant Biol 36 97–124 [DOI] [PubMed] [Google Scholar]
- McElrone AJ, Bichler J, Pockman WT, Addington RN, Linder CR, Jackson RB (2007) Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves. Plant Cell Environ 30 1411–1421 [DOI] [PubMed] [Google Scholar]
- Picaud S, Becq F, Dedaldechamp F, Ageorges A, Delrot S (2003) Cloning and expression of two plasma membrane aquaporins expressed during the ripening of grape berry. Funct Plant Biol 30 621–630 [DOI] [PubMed] [Google Scholar]
- Pilati S, Perazzolli M, Malossini A, Cestaro A, Dematte L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C (2007) Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics 8 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C (1971) Reproductive anatomy in cultivated grapes: review. Am J Enol Vitic 22 92–109 [Google Scholar]
- Rogiers SY, Smith JA, White R, Keller M, Holzapfel BP, Virgona JM (2001) Vascular function in berries of Vitis vinifera (L) cv. Shiraz. Aust J Grape Wine Res 7 47–51 [Google Scholar]
- Shiota H, Sudoh T, Tanaka I (2006) Expression analysis of genes encoding plasma membrane aquaporins during seed and fruit development in tomato. Plant Sci 171 277–285 [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA (2006) Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant Cell Environ 29 993–1001 [DOI] [PubMed] [Google Scholar]
- Tilbrook J, Tyerman SD (2009) Hydraulic connection of grape berries to the vine: varietal differences in water conductance into and out of berries, and potential for backflow. Funct Plant Biol 36 541–550 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Tilbrook J, Pardo C, Kotula L, Sullivan W, Steudle E (2004) Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Aust J Grape Wine Res 10 170–181 [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ieperen W, Volkov VS, Van Meeteren U (2003) Distribution of xylem hydraulic resistance in fruiting truss of tomato influenced by water stress. J Exp Bot 54 317–324 [DOI] [PubMed] [Google Scholar]
- Wada H, Shackel KA, Matthews MA (2008) Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 227 1351–1361 [DOI] [PubMed] [Google Scholar]
- Yamada S, Katsuhara M, Kelly WB, Michalowski CB, Bohnert HJ (1995) A family of transcripts encoding water channel proteins: tissue specific expression in the common ice plant. Plant Cell 7 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP (2006) A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol 142 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Setz N, Niemietz C, Qu H, Offler CE, Tyerman SD, Patrick JW (2007) Aquaporins and unloading of phloem-imported water in coats of developing bean seeds. Plant Cell Environ 30 1566–1577 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291 1059–1062 [DOI] [PubMed] [Google Scholar]