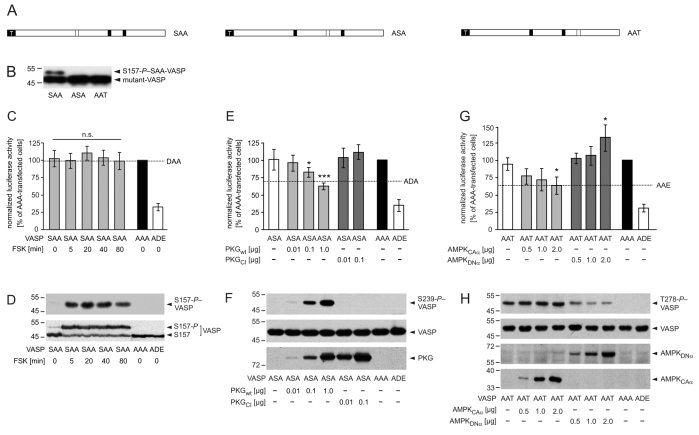
Fig. 8.
Phosphorylation at S239 and T278 but not at S157 impairs VASP-driven actin polymerization in living cells. (A) Schematic representation of partially arrested VASP mutants. To analyze defined VASP phosphorylation patterns, we substituted two of the three phosphorylation sites with alanine residues, retaining one to allow kinase-mediated phosphorylation specifically at this residue. Black and open boxes indicate alanine and an accessible phosphorylation site, respectively. White `T' indicates the tag. (B) Western blot with anti-VASP antibodies to confirm expression of mutants SAA, ASA or AAT in transfected EC_VASP–/– cells. (C-D) PKA-mediated phosphorylation of S157-VASP does not affect global cellular F-actin content. Transfected HEK293 cells were incubated with forskolin (FSK, 5 μM) for 5, 20, 40 and 80 minutes. (C) SRE assay. The luciferase activities in cells transfected with AAA or empty vector (MOCK) were set to 100% and 0%, respectively. For comparison, the luciferase activity of unstimulated cells expressing DAA is given (dashed line, n=6; ANOVA, P>0.05 stimulated versus unstimulated SAA-transfected cells). The transfection with ADE, which maximally blocked VASP-driven actin assembly (Fig. 5B), served as a positive control. (D) The expression of VASP mutants and phosphorylation of SAA at S157 was confirmed by immunoblotting using antibodies against S157-P (upper panel) and total-VASP (lower panel). (E-F) PKG-mediated VASP phosphorylation at S239 reduces global actin polymerization. HEK239 cells were co-transfected with VASP mutant ASA and the indicated amounts of PKGwt or PKGCI. (E) For comparison, the signal of unstimulated cells expressing ADA is given (dashed line). Normalized luciferase activities are plotted relative to the activity in cells transfected with AAA or empty vector (MOCK) (set to 100% and 0%, respectively; n=8; ANOVA, *P<0.05, ***P<0.001). ADE served as a positive control. (F) Anti-S239-P antibodies indicated the phosphorylation of S239-ASA (upper panel) in western blots. The expression of VASP mutants in transfected cells was confirmed by immunoblotting with anti-VASP antibodies (middle panel). The expression of PKG variants was probed using antibodies against PKG (lower panel). (G-H) AMPK-meditated phosphorylation of VASP at T278 interferes with F-actin assembly. HEK239 cells were transiently co-transfected with VASP-AAT and AMPKCAα or AMPKDNα. (G) For comparison, the activity of AAE-transfected cells is indicated (dashed line). Normalized luciferase activities are plotted relative to the activity in cells transfected with AAA or empty vector (MOCK) (set to 100% and 0%, respectively; n=8; ANOVA, *P<0.05). ADE served as a positive control. (H) Western blots using anti-T278-P (upper panel) and anti-VASP antibodies (second panel) confirmed T278 phosphorylation and AAT expression, respectively. AMPK variants were probed by myc-tag specific antibodies (lower panels).