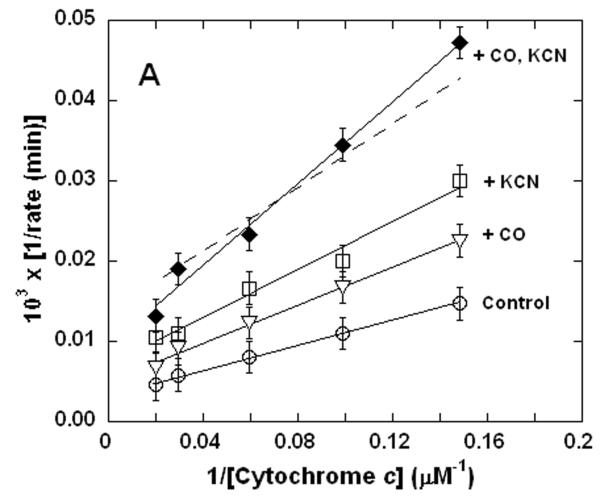
Figure 1. Dual inhibition of cytochrome c oxidase (complex IV) turnover (spectrophotometric measurements) by CO + CN-, NO + CN-, and NO + CO.
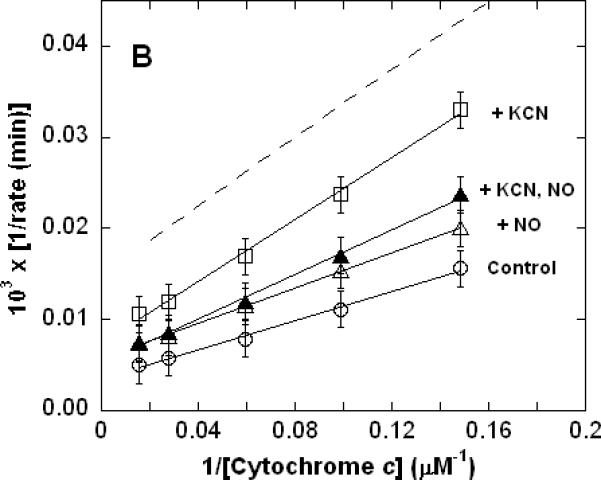
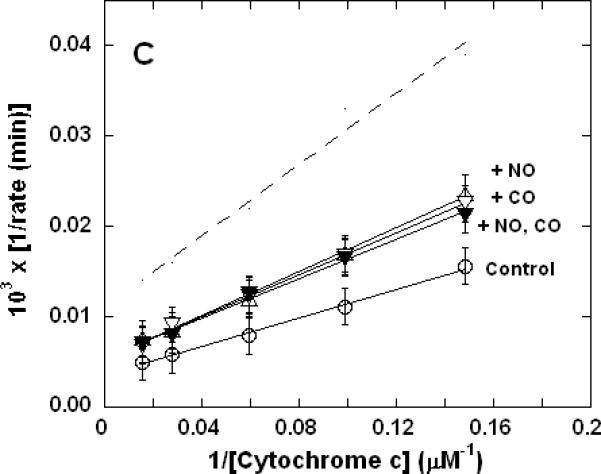
Lineweaver-Burk (double reciprocal) plots showing inhibition of ferrocytochrome c oxidation. Reaction conditions were 1.2 nM enzyme in 0.1 M aqueous potassium phosphate buffer, pH 7.4, 1.0 mM in EDTA, 0.05% in lauryl maltoside, 22 °C. A. Uninhibited control (○), 0.5 mM CO (▽), 50 nM KCN (□), 0.5 mM in CO and 50 nM in KCN (◆); B. uninhibited (○), 0.5 μM NO (Δ), 50 nM KCN (□), 50 nM in KCN and 0.5 μM in NO (▲); C. uninhibited (○), 0.5 mM CO (▽), 0.5 μM NO (Δ), 0.5 μM in NO and 0.5 mM in CO (▼). In each panel, the broken line represents the combined effect of the two relevant inhibitors predicted by simple summation of their individual measured effects.