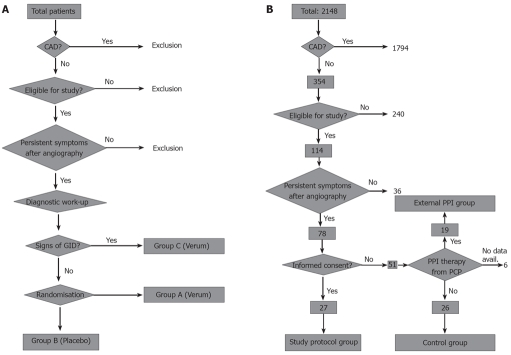
Figure 1.
Study design. A: Original study design with 3 patient groups. Randomization was planned after the diagnostic procedures in order to randomize only patients without overt signs of gastrointestinal disease. After recruitment of the first 10 patients only for patient group C, the other two patient groups were closed and the study design was adapted as depicted in B (CAD: Coronary artery disease; GID: Gastrointestinal disease); B: Adapted study design without randomization. To compare the results of per protocol treatment to patients treated in a non-standardized fashion or untreated patients, an external PPI group and a control group were built by recruiting patients who were treated by their primary care physicians (external PPI group, 19 patients) or were not willing to undergo treatment at all (control group, 26 patients). These patients were not matched to the study protocol group and most of them did not receive diagnostic procedure regarding gastrointestinal diseases (CAD: Coronary artery disease; PCP: Primary care physician).