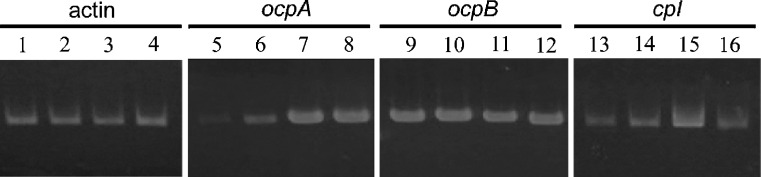
Fig. 7.
Semiquantitative RT-PCR analysis of ocpA, ocpB, and cpI in A. oryzae RIB40. mRNA was isolated from approximately 0.1 g (semidry wet) of mycelia cultured for 1 day in CDSN (lanes 1, 5, 9, 13), CDGN (lanes 2, 6, 10, 14), CDGM (lanes 3, 7, 11, 15), or CDGMN (lanes 4, 8, 12, 16) media. Each medium contained different carbon and nitrogen sources. CDSN contained 3% starch and 0.3% NaNO3, CDGN contained 3% glucose and 0.3% NaNO3, CDGM contained 3% glucose and 0.3% skim milk, and CDGMN contained 3% glucose, 0.3%NaNO3, and 0.3% skim milk. PCR for amplification of ocpA, ocpB, and cpI was performed using 32 cycles and that for γ-actin was performed using 26 cycles. The PCR amplification of γ-actin using each cDNA as a template was carried out for quantitative control. Negative-control PCR analysis using each mRNA as a template showed no amplified fragments (data not shown)