Abstract
Objective
Longitudinal follow-up of neurocognitive functioning in people with pediatric bipolar disorder (PBD) was conducted to characterize the developmental trajectory of cognitive disabilities in this disorder.
Method
Patients with PBD (n = 26) and controls (HC; n = 17; mean age 11.66 ± 2.70 years) completed cognitive testing at baseline and then again at a 3-year follow-up. Groups were matched at baseline on age, sex, race, parental socioeconomic status, general intelligence, and single-word reading ability. The PBD group received treatment guided by a standardized medication algorithm during the 3-year period. A battery of neuropsychological tests was administered to assess attention, executive function, working memory, verbal memory, visual memory, and visuospatial perception at baseline and follow-up.
Results
At baseline and follow-up, the patients showed deficits in all of the examined domains. At 3-year follow-up, developmental progress in executive functions and verbal memory was significantly less in the patients with PBD than in the HC. Improvement on attention, working memory, visual memory, and visuospatial perception tasks in the patients with PBD was comparable to that of the HC, but the patients with PBD remained impaired in all domains relative to the HC.
Conclusions
The developmental delay in some neurocognitive functioning in PBD suggests that the illness disrupts cognitive development with potential lifelong implications for reduced functional ability. Treating bipolar symptoms does not seem to prevent the lag in cognitive development. This dysmaturation may be a direct effect of the illness on brain function, or it may represent indirect consequences of psychopathology or medications on cognitive development.
Keywords: bipolar, neurocognitive, development, cognition
Pediatric bipolar disorder (BD) is a disabling illness associated with neurocognitive deficits, affect dysregulation, a high incidence of suicidal behavior, substance abuse, and academic failure.1–3 Although cognitive deficits in pediatric BD (PBD) are now established,4–10 little is known about their developmental trajectory. Either because of a direct effect of the disorder on brain function or because of the disorder’s interference with school-based learning, it is possible that PBD could disrupt the typical developmental cognitive trajectory with a potential lifelong impact of reduced functional ability.
Cognitive domains that are implicated in PBD include attention, working memory, executive function, verbal memory, response flexibility, reversal learning, processing speed, set shifting, and visuospatial memory.4–10 In patients with PBD, symptomatic treatment for mania does not seem to alleviate the “traitlike” cognitive impairments. Regardless of whether patients with PBD are medicated and euthymic, or untreated and acutely ill, attention, working memory, executive function, and verbal memory seem to remain impaired.9 Furthermore, global neurocognitive deficits have been associated with reading difficulties in patients with PBD, and impaired vigilance has been linked to math difficulties.5,11,12 These learning difficulties, often associated with poor academic achievement, lead to greater use of special education services by patients with PBD.11,12 Therefore, it is imperative that we gain further understanding of the developmental trajectory of persistent cognitive dysfunction in PBD to develop early and more effective interventions.
At present, although cognitive deficits in PBD have been established, it is not clear whether children with PBD have a static deficit relative to their peers, whether they eventually catch up with their grade level of academic achievement after sustained treatment, or whether they have a slowed rate of cognitive development after illness onset that leaves them progressively farther behind their typically developing peers. Cahill et al.13 recently reviewed neuropsychological research in adult BD and compared the findings with emergent data on neuropsychological function in PBD, which revealed that there are virtually no longitudinal studies in adult BD just as is the case in PBD. However, problems in working memory, attention, and executive function domains are found in euthymic state among both the adult BD and PBD patient groups, suggesting that the neurocognitive deficits extend across a wide age range and that they seem to persist regardless of remission in symptoms.10,14 However, the emergence, longitudinal trajectory, and degree of functional impairment due to neurocognitive deficits are unknown.
In the present study, we characterized neurocognitive development in the domains of executive function, attention, working memory, verbal memory, visual memory, motor skills, and visuospatial perception in patients with PBD relative to controls (HC). Given the existing cross-sectional neuropsychological findings, neurodevelopmental nature of PBD, and the ongoing changes in brain structure and function in youths,15 we hypothesized that there would be delays in cognitive development in patients with PBD versus HC evaluated during a 3-year follow-up period. Our data analysis strategy was to establish baseline deficits, then test for omnibus deficit in cognitive development during the follow-up period, and to follow up significant overall differences in cognitive development profile with step-down cognitive domain-wise comparisons of PBD and HC. The aim of these latter analyses was to determine whether patients with PBD catch up with their peers without PBD or whether disparity in cognitive development widens the cognitive differences between groups, and whether effects were consistent or different across cognitive domains. Of note, the patients were mostly euthymic at baseline testing and were treated during the 3 years between baseline and follow-up testing with a medication algorithm.16
METHOD
Subjects
The study sample included 43 youths aged 11.66 (±2.70) years at the time of recruitment. There were 26 subjects with PBD and 17 HC, matched on age, sex, race, and estimated premorbid intellectual functioning (Table 1). The patients with PBD were recruited from our clinic during a 6-month period, January to June 2004. In view of our interest in providing clinical follow-up via a medication algorithm during the study period, we enrolled only subjects living within a 2-hour drive from the University clinic who were eligible to participate. At baseline, 1 subject with mania, 2 subjects with hypomania, and 23 subjects in euthymic phase were included in the PBD group. The symptomatic patients were relatively treatment resistant (failed three trials of mood stabilizers at the time of entry into our longitudinal study). All of the patients went on to receive treatment consistent with an updated evidence-based medication algorithm within our clinical program.16,17 The medication algorithm is available online at http://ccm.psych.uic.edu/Research/ResearchProgram/MoodDisorder/PavuluriAlgorithm.pdf. Using this method, we avoided polypharmacy where possible to minimize cognitive deficits. Second, we aimed to maximize chances of maintaining clinical recovery by following effective and evidence-based pharmacotherapy so that residual symptoms would be minimal and not account for cognitive deficits. The clinical status of the patients at baseline and follow-up is summarized in Table 1, and the medications are summarized in Table 2. The HC group had no DSM-IV diagnosis or family history of BD (Table 1). Exclusion criteria for the PBD and HC group were active substance abuse, serious medical problems, or an IQ of lower than 70 as determined by the Wechsler Abbreviated Scale of Intelligence.18 This study was approved by the institutional review board at the University of Illinois at Chicago. Verbal or written assent was obtained from all of the subjects in addition to written consent from parents.
TABLE 1.
Demographic and Clinical Characteristics of the HC and Patients With PBD at Baseline and at 3-Year Follow-up
| Baseline |
Follow-up |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC |
PBD |
HC |
PBD |
|||||||
| Mean | SD | Mean | SD | F | Mean | SD | Mean | SD | F | |
| Age, y | 11.4 | 2.5 | 11.9 | 2.9 | F2,41 = 1.6 | 14.5 | 2.6 | 14.3 | 2.9 | F1,41 = 0.1 |
| SES | 1.3 | 0.6 | 1.5 | 0.7 | F2,41 = 1.4 | |||||
| YMRS | 0.2 | 0.5 | 11.9 | 9.1 | F2,41 = 64.1* | 0.8 | 1.1 | 8.6 | 3.1 | F1,41 = 61.4* |
| CDRS-R | 18.4 | 0.8 | 29.5 | 14.8 | F2,41 = 16.1* | 18.7 | 1.8 | 28.8 | 10.3 | F1,41 = 45.1* |
| WASI | 111.8 | 6.8 | 108.7 | 11.7 | F1,41 = 0.1 | |||||
| WRAT | 106.1 | 10.67 | 103.6 | 9.5 | F2,41 = 0.1 | |||||
| n | % | n | % | χ2 | df | F | ||||
| Sex | ||||||||||
| Male | 6 | 35.3 | 17 | 65.4 | 0.2 | 1 | ||||
| Female | 11 | 64.7 | 9 | 34.6 | ||||||
| Race | ||||||||||
| White | 7 | 41.2 | 20 | 76.9 | 2.14 | 1 | ||||
| Other | 10 | 58.8 | 6 | 23.1 | ||||||
| ADHD Comorbid | 0 | 0 | 16 | 65.5 | 2.1 | 1 | ||||
| Handedness | ||||||||||
| Right | 16 | 94.1 | 25 | 96.2 | F1,40 = 0.1 | |||||
| Left | 1 | 5.9 | 1 | 3.9 | ||||||
Note: ADHD = attention-deficit/hyperactivity disorder; CDRS-R = Children’s Depression Rating Scale–Revised; HC = healthy controls; PBD = pediatric bipolar disorder; SES = socioeconomic status; WASI = Wechsler Abbreviated Scale of Intelligence; WRAT = Wide Range Achievement Test; YMRS = Young Mania Rating Scale.
p < .001.
TABLE 2.
Medications Used to Treat Patients With Pediatric Bipolar Disorder
| Type of Medication | Baseline, n (%) | Follow-up, n (%) |
|---|---|---|
| Mood stabilizers (lithium, divalproex sodium, oxcarbazepine, lamotrigine) | 26 (73) | 18 (69) |
| Second-generation antipsychotics (risperidone, quetiapine, aripiprazole) | 6 (23) | 7 (26) |
| Psychostimulants (methylphenidate long-acting form, mixed amphetamine salts long-acting form) | 13 (50) | 15 (57) |
| Serotonin reuptake inhibitors | 0 (0) | 8 (30) |
| Guanfacine | 1 (4) | 5 (19) |
| No medications | 4 (15) | 2 (7) |
Procedure
Each subject and at least one of their parents were interviewed using the Washington University, St. Louis, Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic version (WASH-U-K-SADS).19 Interrater reliability for diagnosis among the research interviewers at the end of training (PBD versus HC) was 0.98 by Cohen − for the diagnosis on the WASH-U-K-SADS. The final lifetime DSM-IV diagnosis was made based on consensus gained from independent clinical interview, other available clinical data, and ratings on the WASH-U-K-SADS. The WASH-U-K-SADS interviews, as well as the Young Mania Rating Scale (YMRS)20 and Children’s Depression Rating Scale-Revised (CDRS-R),21 were completed by raters with no knowledge of cognitive test performance data.
Intellectual and neuropsychological testing was performed at baseline and repeated at 3-year follow-up by postdoctoral-level neuropsychologists. The battery consisted of the Wechsler Abbreviated Scale of Intelligence, the Trail Making Test, the Digit Span subtest from the Weschler Memory Scale-Third Edition, and the California Verbal Learning Test-Child version.22 The computerized component of the battery was compiled from the University of Pennsylvania Computerized Neuropsychological Battery23 and Cogtest.24 Tests selected from the Penn battery included the Penn Conditional Exclusion Test, the Penn Continuous Performance Test, the Penn Face Memory Test, and the Penn Computerized Judgment of Line Orientation. Tests selected from the Cogtest battery included the Set Shifting Test and the Controlled Oral Word Association Test.
Statistical Analysis
To provide a standard metric for comparison across neurocognitive domains, tests scores were standardized (i.e., converted to z scores) relative to the baseline performance of the control comparison group. Extreme z scores for individual test scores were truncated to ±3.0 to avoid their undue impact on group comparisons. A z score of ±3 was chosen as a cutoff value to allow sufficient variability in the data while limiting the impact of the few extreme scores. Extreme negative scores were noted for two cases at baseline (on verbal memory and working memory) and 6 cases at follow-up (on verbal memory, visual memory, and visuospatial perception). A total of 10 raw test scores, 2 at baseline and 8 at follow-up, were truncated.
Standardized domain scores were then calculated for executive function, attention, working memory, verbal memory, visual memory, motor skills, and visuospatial perception by combining normalized z scores (standardized to baseline performance of the controls) from tests assessing each of the six cognitive domains (Table 3). Internal consistencies of scores comprising each neurocognitive domain were calculated using Chronbach α and were reported in a previous article.9 A global index of neurocognitive functioning was calculated by averaging the six cognitive domain scores for each subject.
TABLE 3.
Performance of Patients With PBD and HC on Individual Neuropsychological Tests Organized by Cognitive Domains of Interest (Raw Scores)
| Neuropsychological Domain | HC Baseline | HC Follow-up | PBD Baseline | PBD Follow-up | Effect Sizeb/p |
|---|---|---|---|---|---|
| Attention | |||||
| Trail Making Test: Part A time | 25.11 ± 7.82 | 23.76 ± 7.88 | 42.46 ± 18.44 | 32.77 ± 15.33 | |
| Penn Continuous Performance Test | |||||
| True positives | 33.56 ± 3.10 | 35.00 ± 1.00 | 28.31 ± 8.79 | 33.15 ± 2.95 | |
| False positives | 8.13 ± 4.90 | 4.47 ± 4.02 | 23.04 ± 29.71 | 7.85 ± 7.96 | |
| Domain za score | −0.01 ± 0.59 | 0.58 ± 0.54 | −0.91 ± 0.88 | −0.05 ± 0.89 | 0.44 |
| Working memory | |||||
| WMS-III: Digit Span (raw) | 14.71 ± 2.49 | 17.59 ± 3.55 | 13.42 ± 3.40 | 15.31 ± 3.40 | |
| WMS-III: Spatial Span (raw) | 15.88 ± 3.20 | 17.18 ± 3.76 | 12.85 ± 3.52 | 14.81 ± 3.36 | |
| Domain za score | 0.00 ± 0.77 | 0.60 ± 1.00 | −0.76 ± 1.34 | −0.18 ± 0.90 | 0.11 |
| Executive function | |||||
| Cogtest Set Shifting Test | |||||
| Total errors | 15.53 ± 10.68 | 4.59 ± 3.45 | 37.85 ± 36.91 | 30.00± 32.27 | |
| Penn Conditional Exclusion Test | |||||
| Total errors | 23.65 ± 16.26 | 14.29 ± 7.75 | 24.62 ± 12.12 | 24.81 ± 17.42 | |
| Trail Making Test: Part B time | 73.59 ± 28.65 | 57.35 ± 19.21 | 100.46 ± 41.23 | 89.35 ± 33.30 | |
| Controlled Oral Word Association | |||||
| Letters (mean C) | 10.06 ± 4.73 | 12.29 ± 4.03 | 9.00 ± 4.38 | 9.96 ± 3.35 | |
| Letters (mean F) | 9.63 ± 3.18 | 12.53 ± 3.02 | 9.27 ± 4.54 | 10.08 ± 3.44 | |
| Letters (mean L) | 9.75 ± 3.70 | 11.47 ± 3.00 | 8.92 ± 3.74 | 9.35 ± 3.37 | |
| Categories (mean animal) | 21.56 ± 5.80 | 21.65 ± 4.53 | 16.58 ± 5.87 | 18.31 ± 6.92 | |
| Categories (mean fruit) | 17.00 ± 5.02 | 19.00 ± 4.56 | 13.08 ± 5.80 | 15.08 ± 5.78 | |
| Domain za score | −0.02 ± 0.60 | 0.64 ± 0.21 | −0.59 ± 0.87 | −0.37 ± 0.80 | 0.79* |
| Visual memory | |||||
| Penn Face Memory Test: immediate recognition | 28.47 ± 4.64 | 33.25 ± 2.32 | 26.88 ± 4.27 | 30.08 ± 3.68 | |
| Penn Face Memory Test: delay recognition | 32.07 ± 3.54 | 35.41 ± 2.12 | 26.64 ± 7.65 | 31.19 ± 4.84 | |
| Domain za score | −0.03 ± 0.74 | 0.99 ± 0.44 | −0.80 ± 1.10 | 0.05 ± 1.04 | 0.24 |
| Verbal memory | |||||
| CVLT: total trials 1–5 (raw) | 54.24 ± 5.63 | 58.47 ± 7.10 | 46.46 ± 8.15 | 45.19 ± 11.86 | |
| CVLT: short delay free recall | 10.94 ± 1.89 | 12.35 ± 1.77 | 9.84 ± 2.95 | 9.54 ± 2.89 | |
| CVLT: long delay free recall | 11.53 ± 1.70 | 12.29 ± 1.72 | 10.35 ± 2.68 | 10.46 ± 2.40 | |
| Domain za score | 0.00 ± 0.90 | 0.65 ± 0.98 | −0.77 ± 1.20 | −0.85 ± 1.32 | 0.75* |
| Visuospatial perception | |||||
| Penn Computerized Judgment of Line Orientation | |||||
| Total correct | 20.94 ± 4.94 | 24.18 ± 4.13 | 17.19 ± 4.73 | 18.42 ± 4.51 | |
| Domain za score | 0.00 ± 1.00 | 0.66 ± 0.84 | −0.76 ± 0.96 | −0.51 ± 0.91 | 0.55 |
| Global functioning (za score) | −0.01 ± 0.52 | 0.69 ± 0.35 | −0.76 ± 0.76 | −0.32 ± 0.68 | 0.67* |
Note: Domain scores are normalized to baseline data from the control subjects. CVLT = California Verbal Learning Test-Second Edition; HC = healthy controls; PBD = pediatric bipolar disorder; WMS-III = Wechsler Memory Scale-Third Edition.
Composite z scores reflecting performance across tests in a domain.
Effect size in units of Cohen d for differential pre-post change between HC and PBD.
p < .05.
Data Analysis Plan
Given the number of cognitive outcome measures, we began data analysis with an omnibus test comparing the PBD and HC groups on the global index of neurocognitive performance. In the presence of significant group difference on the global neuropsychological measure, step-down domain-wise analyses were undertaken. This was done for baseline data and to test the significance of differential group change in cognitive performance during the 3-year follow-up period. The central question guiding these analyses was whether long-term treatment for PBD would repair or attenuate deficits in cognitive functioning observed at baseline and allow these children to catch up with their peers without PBD or whether the disorder would lead to an increasing cognitive disparity relative to peers without PBD. This question was evaluated using repeated-measures analysis of variance (ANOVA) to test the significance of the group-by-time interaction in an omnibus test on the cognitive composite score, followed by similar domain-specific analyses. The group-by-time interaction effect was used to test whether there was a differential rate of change in neurocognitive function over time in the PBD group compared with the controls.
RESULTS
Demographic and Clinical Features
The scores on the YMRS and CDRS-R are summarized in Table 1. Repeated-measures analyses revealed no significant change in YMRS or CDRS-R scores between the two assessment periods in either the PBD or the HC groups. Medications were prescribed as guided by our pharmacotherapy algorithm,16 with the first step being the use of a mood stabilizer with or without an antipsychotic. Medications used in the treatment paradigm are summarized in Table 2. There are no significant differences in percentages of mood stabilizers, second-generation antipsychotics or psychostimulants used by patients at baseline and follow-up. Psychostimulants were used in 15 of the 16 subjects with PBD who had comorbid attention-deficit/hyperactivity disorder (ADHD). One subject with comorbid ADHD had a history of worsening clinical state on stimulants and was not prescribed a stimulant. Two subjects had significantly increased manic symptoms on psychostimulants during the course of treatment, and therefore, the adjunctive treatment was discontinued. Two additional subjects had severe inattention in euthymic state warranting stimulant medication despite having no formal diagnosis of ADHD before 7 years of age. Therefore, overall, 15 patients with PBD were treated with stimulants throughout the 3-year study.
Baseline Neurocognitive Functioning
First, to establish baseline deficits in subjects with PBD, a one-way ANOVA was conducted to test for group differences on the global neuropsychological performance index at baseline. Results indicated that the PBD group performed significantly more poorly than the HC group (F1,41 = 12.6, p < .001). Given the significant global deficit at baseline, univariate ANOVAs were computed to evaluate function in each neuropsychological domain. Results indicated that children in the PBD group, relative to HC, showed impairment on all six domains: executive function (F1,41 = 5.6, p < .05), attention (F1,41 = 13.6, p < .001), verbal memory (F1,41 = 5.4, p < .05), visual memory (F1,41 = 6.1, p < .05), visuospatial perception (F1,41 = 6.2, p < .05), and working memory (F1,41 = 4.5, p < .05). These findings are consistent with our previous report of impairment in patients with PBD in all domains in a smaller sample.11
Longitudinal Analyses of Neurocognitive Functioning
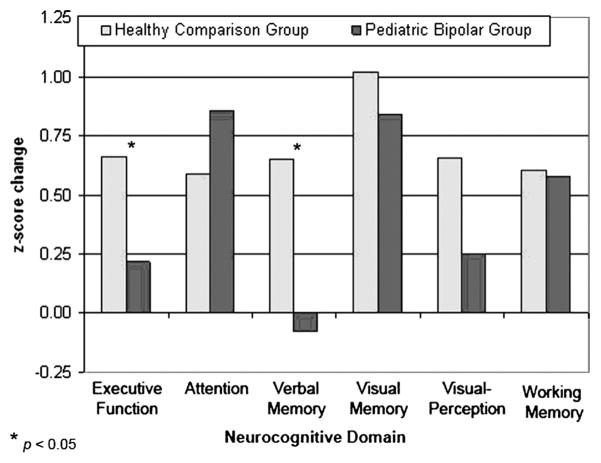
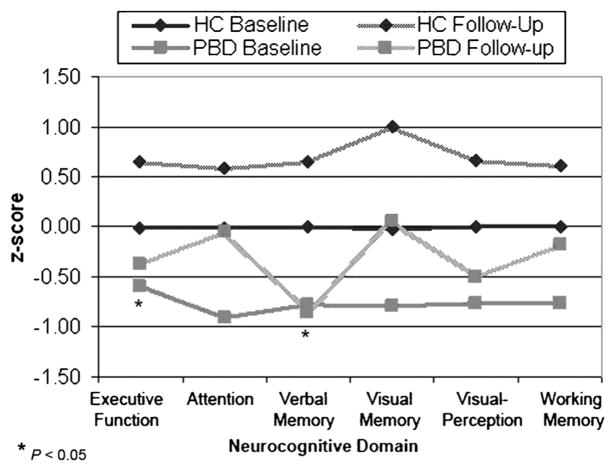
A two-way repeated-measures ANOVA on the global index revealed a significant time-by-group interaction (F1,41 = 4.4, p < .05), with the PBD group improving at a slower rate over time than the HC group. In the presence of significant omnibus findings for longitudinal data, univariate longitudinal ANOVA were computed to evaluate patient versus control differences in cognitive development in each domain. Level of neuropsychological deficit is illustrated in Figure 1 for both time points. Although the step-down analyses were “protected” by the omnibus analysis, we used a critical value of p < .025 for these planned univariate ANOVA. As seen in Figure 2 and Table 3, the results indicated differential cognitive change in patients and controls during the 3-year follow-up period for executive function (F1,41 = 6.1, p < .05) and verbal memory (F1,41 = 5.5, p < .05). On the executive function domain, children in both groups improved during 3 years, but the HC showed significantly greater improvement from baseline performance (z score change of 0.67) than the PBD group (z score change of 0.22), thus widening what was already a performance deficit in the PBD group at baseline testing. In the case of verbal memory, the performance of children in the PBD group actually showed a slight decrease during 3 years (z score change of −0.08), whereas the HC showed significant improvement during the same period (z score change of 0.65). Improvement in the PBD and the HC groups from baseline to follow-up did not differ for the visual memory, visuospatial perception, and working memory domains. Analyses with follow-up data, similar to those at baseline, revealed significant deficits in the subjects with PBD relative to HC in all cognitive domains including attention (F1,41 = 4.1, p < .01), working memory (F1,41 = 7.2, p < .01), executive function (F1,41 = 26.1, p < .001), verbal memory (F1,41 = 16.1, p < .001), visual memory (F1,41 = 12.6, p < .001) and visuospatial perception (F1,41 = 17.9, p < .001).
Fig. 1.
Mean z scores of patients with pediatric bipolar disorder for each neurocognitive domain. Performance on each domain at baseline is normed to that of age-matched control peers. Similarly, performance on follow-up on each of the domains is standardized relative to the control group at follow-up.
Fig. 2.
Change in standardized neurocognitive function at the 3-year follow-up relative to baseline performance for patients with pediatric bipolar disorder and controls.
Change in cognitive function was not predicted by YMRS and CDRS-R scores at baseline or follow-up for any domain. Within the patient group, there was no significant difference in change on attention test performance among those who received stimulants and those who did not. Excluding the patients that presented with mania or hypomania at baseline (n = 3) did not change any of the study findings.
Relation to Academic Functioning
To assess the relation between academic function and neuropsychological performance, we evaluated parent reports of general indices of academic function (e.g., repeated grade, special education/services, significant difficulty with early writing or math, and overall quality of grades). These data were measured using categorical outcomes (whether parents reported a significant problem); therefore, post hoc analysis of longitudinal change in these indices was assessed using a χ2 test for differential change over time. There was no significant change over time for the HC for any of the indices measured. However, the PBD group showed an increase from 35% of patients with PBD having math difficulties at baseline to 62% at follow-up (χ2 = 8.1, p < .05). In assessing the association between math problems and the two neurocognitive domains (verbal memory and executive function) showing differential change during the follow-up period, the PBD group was divided into two groups: those with parental reports of “math difficulties”(n = 16) and those whose parents did not report math difficulties (n = 10) at the follow-up assessment. Repeated-measures ANOVA revealed that children in the PBD group whose parents reported math difficulties also had significantly lower verbal memory scores at follow-up (approximately a full standard deviation below the patients with PBD that had no math problems; F1,24 = 11.3, p < .01). Interestingly, neither the “math difficulties” nor the “no math difficulties” PBD groups showed significant improvement in verbal memory over the course of the study. Thus, preexisting verbal memory dysfunction in patients with PBD may be directly or indirectly related to a risk factor for later emerging math problems in this population.
DISCUSSION
This study is the first study to chart the profile of cognitive development in PBD. The central findings of this 3-year longitudinal study of neurocognitive function are that, even with ongoing evidence-based pharmacological management, executive function, attention, verbal memory, visual memory, visuospatial perception, and working memory continued to be impaired in the PBD group relative to typically developing peers and that there was a slower rate of cognitive development in the PBD group in the areas of executive function and verbal memory. There was no difference in the rate of developmental progression during the 3-year follow-up period in the attention, working memory, visual memory, or visuospatial perception domains, but deficits were still evident in all cognitive domains at follow-up. Whereas this documents a typical rate of cognitive development in these four domains, it also indicates a lack of ability of the PBD group to catch up with the level of functioning in the control comparison group at the end of the 3-year follow-up.
Individual domains of cognition varied in their degree of developmental success in subjects with PBD. Executive functions were examined using tests of set shifting (cognitive flexibility), problem solving, and word generation. Our results are consistent with those of Dickstein and coworkers7 who reported impaired simple reversal learning and shifting to an alternative non-prepotent response in narrow phenotype patients with PBD. Henin and coworkers5 also reported poor executive skills and processing speed in patients with PBD, postulating a potential adverse impact on functional abilities. Our findings build on cross-sectional findings5,7,25 by showing that patients with PBD have a slower rate of cognitive development through adolescence that leaves them slipping further behind their peers in cognitive ability. Furthermore, our data show that this effect of reduced cognitive development extended to the domain of verbal memory.
Deficits in executive function have been reported previously in adult BD,26,27 illustrating a continuity in this deficit across pediatric and adult variants of the disorder. To the extent that our developmental findings can be projected forward, our findings raise the possibility that executive deficits may be more pronounced in adult life when BD has its onset in late childhood or early adolescence.
The prefrontal cortex (PFC), which is central to executive control, continues to develop throughout adolescence and into early adulthood. From a functional perspective, the PFC is one of the last regions of the brain to mature, which is reflected in parallel developmental progression in the areas of executive abilities such as voluntary response inhibition that typically do not mature until age 15 to 16 years.28,29 Our neuropsychological findings indicating executive function dysmaturation in PBD are consistent with functional imaging evidence of altered PFC function. The ventrolateral PFC and dorsolateral PFC dysfunction seen in youths with PBD relative to HC detected in functional magnetic resonance imaging studies30–33 may underlie the abnormalities in executive function observed in the present study. Real-life impairments associated with PBD such as difficulty transitioning from one activity to the other, organizing and planning behavior, and problem solving may in part be explained by disturbances in executive function. Recognizing the possibility of disrupted cognitive developmental trajectories in PBD may be an important initial step toward implementing supplemental educational interventions that may limit the long-term academic and functional impact of PBD.
Verbal memory impairment is the most consistent finding across previous cross-sectional neurocognitive studies in PBD4,8,9,34 and may be caused by altered integration of frontal and mesial temporal systems. Indeed, dynamic mapping of structural cortical development during the course of the evolution of bipolar diathesis in psychotic patients demonstrates increasing frontotemporal abnormalities.35,36 Our results indicated a robust failure of cognitive development of verbal memory abilities in patients with PBD and that this deficit was related to math difficulties. Indeed, verbal memory capacity, which is related to phonological processing and processing speed abilities, has been directly linked to arithmetic calculation skills in children.37–39 To stop the decay in speech-based information storage that is important for more complex arithmetic, vocal and subvocal rehearsal of verbal information serves as a building block to counting, multiplication, calculation, and problem solving.40,41 Given that this type of verbal learning is more pronounced in early stages of development,42 early interventions in classrooms may be valuable for an at-risk population such as PBD. Computer-assisted instruction43 and related “attention process training”44 that offer a combination of verbal memory, attentional, and arithmetic problem solving skills seem to be promising, and such pilot studies are under way in our laboratory.
The rate of developmental progression in the domains of attention, visual memory, and working memory in the PBD group were comparable to that in the HC. To some extent, although this could be attributed to the consistent use of psychostimulant medication used to treat comorbid ADHD during the 3-year follow-up period for those who needed or tolerated them,16 our results did not show a significant difference in attention between those who received stimulants and those who did not. Indeed, stimulant treatment for those with ADHD and inattention problems (58% of the patients) did not alleviate the attention problems in the PBD group, who still lagged behind the typically developing children.
The results in the PBD group with regard to persistent attention deficits are consistent with earlier findings from cross-sectional studies in PBD4,6,9 and adult BD.45–47 Recent functional magnetic resonance imaging studies in patients with PBD investigating sustained attention and response inhibition demonstrated frontostriatal abnormalities in adolescent bipolar youths.31,48 Persistent frontostriatal abnormalities in part may help explain the enduring attentional difficulties in patients with PBD. Future studies with a larger sample size are required to tease out the nature of attentional problems and associated circuitry in patients having PBD with and without comorbid ADHD.
Similar to the attentional domain, working memory showed cognitive development in the PBD group at a rate similar to that in the HC, but patients were left with a similar deficit relative as was present at baseline. It may be that the appropriate medication regimen for PBD is partly effective in facilitating recruitment of attentional and working memory systems to prevent an illness-related developmental slowing in these domains. Visual memory and visuospatial perception, although showing comparable progress as the HC, also remained areas of dysfunction at 3-year follow-up.
The present findings of a developmental delay in executive function and verbal memory are novel and potentially have considerable clinical and prognostic significance. Nevertheless, additional work is needed to explain the cause of the developmental delay. One possibility is that such delays represent a direct manifestation of illness-related neurobiology on the maturation of prefrontal and mesial temporal brain systems. A second possibility is that the illness may reduce interest or involvement in academic activities, resulting in disengaging from the verbal learning, organization, and problem solving in the classroom. Third, in this long-term clinical study, medication treatments used during the follow-up period may have had an impact on the performance on our neurocognitive tests or the development of the cognitive abilities. We implemented a medication algorithm in our clinical program specializing in patients with PBD to provide consistent and state-of-the-art treatment.16 However, the potential impact of these treatments on cognitive development is difficult to determine, given the present knowledge. On the one hand, whereas short-term treatment effects on cognition do not seem to be robust in PBD,9 longer term use of mood-stabilizing drugs may directly minimize illness effects on the biological substrate of cognitive development in adolescence. On the other hand, it may help overall psychological functioning to facilitate a more effective participation in academic activities and thus minimize what would otherwise be a poorer or more generalized pattern of reduced cognitive development. Stimulants may also have had a positive influence in this regard by facilitating school-related learning. Addressing the potential effects of medication treatments for PBD on cognitive development will require more careful attention in future controlled studies.
The strength of this study rests in its demonstration that BD in childhood or early adolescence is associated with reduced cognitive development, even in the context of ongoing state-of-the-art treatment in a specialized research clinic. Future studies are needed to further explain the altered neurodevelopmental trajectory of this disorder. This needs to be investigated by examining parallel changes in brain function and anatomy by examining school performance and its potential mediation of reduced cognitive development and by evaluating whether the effect can be reduced by using different treatments for PBD or potentially by the addition of procognitive adjunctive medications. One of the limitations of the study may be the effects of medication at study entry on baseline cognitive performance. Effects of medication during the follow-up on cognition, as well as at baseline, cannot be ruled out.
To summarize, our results indicate that cognitive functioning remains compromised in PBD despite symptom remission with optimal pharmacotherapy and that cognitive development of executive functions and verbal memory is specifically impaired. These factors may explain the persistent cognitive deficits shown in previous cross-sectional studies of PBD and set the stage for chronically reduced cognitive functioning into adulthood.
Acknowledgments
This study was supported by NIH/NCRR K23 and RR018638-01.
Footnotes
Disclosure: Dr. Pavuluri has received support from the NARSAD Independent Investigator Award, NICHD, Colbeth Foundation, GlaxoSmithKline-NeuroHealth, Abbott Pharmaceuticals, and Janssen Research Foundation. Dr. Sweeney has received support from the NIH, GlaxoSmithKline, AstraZeneca, and Eli Lilly. The other authors report no conflicts of interest.
References
- 1.Geller B, Zimerman B, Williams M, DelBello MP, Frazier J, Beringer L. Phenomenology of prepubertal and early adolescent bipolar disorder: examples of elated mood, grandiose behaviors, decreased need for sleep, racing thoughts and hypersexuality. J Child Adolesc Psychopharmacol. 2002;12:3–9. doi: 10.1089/10445460252943524. [DOI] [PubMed] [Google Scholar]
- 2.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 3.Wilens TE, Biederman J, Kwon A, et al. Risk of substance use disorders in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1380–1386. doi: 10.1097/01.chi.0000140454.89323.99. [DOI] [PubMed] [Google Scholar]
- 4.Doyle AE, Wilens TE, Kwon A, et al. Neuropsychological functioning in youth with bipolar disorder. Biol Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Henin A, Mick E, Biederman J, et al. Can bipolar disorder-specific neuropsychological impairments in children be identified? J Consult Clin Psychol. 2007;75:210–220. doi: 10.1037/0022-006X.75.2.210. [DOI] [PubMed] [Google Scholar]
- 6.Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 7.Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 8.McClure EB, Treland JE, Snow J, et al. Memory and learning in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:461–469. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- 9.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 10.Bearden CE, Glahn DC, Monkul ES, et al. Sources of declarative memory impairment in bipolar disorder: mnemonic processes and clinical features. J Psychiatr Res. 2006;40:47–58. doi: 10.1016/j.jpsychires.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Pavuluri MN, O’Connor MM, Harral EM, Moss M, Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: a clinical translation. Biol Psychiatry. 2006;60:951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry. 1995;34:867–876. doi: 10.1097/00004583-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Cahill CM, Green MJ, Jairam R, Malhi GS. Do cognitive deficits in juvenile bipolar disorder persist into adulthood? J Nerv Ment Dis. 2007;195:891–896. doi: 10.1097/NMD.0b013e318159288b. [DOI] [PubMed] [Google Scholar]
- 14.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 15.Pavuluri MN, Passarotti AM. Neural bases of emotion processing in pediatric bipolar disorder. Expert Rev Neurother. 2008;8:1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 16.Pavuluri MN, Henry DB, Devineni B, Carbray JA, Naylor MW, Janicak PG. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:859–867. doi: 10.1097/01.chi.0000128790.87945.2f. [DOI] [PubMed] [Google Scholar]
- 17.Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Brace & Company; 1999. [Google Scholar]
- 19.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 21.Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Los Angeles: Western Psychological Services; 1995. [Google Scholar]
- 22.Delis DC, Kramer JH, Kaplan E, Ober A. California Verbal Learning Test-Children’s Version (CVLT-C) Manual. San Antonio: Psychological Corporation; 1994. [Google Scholar]
- 23.Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 24.Standardisation and Cross-validation Study of Cogtest an Automated Neurocognitive Battery for Use in Clinical Trials. 2002 [Google Scholar]
- 25.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 26.Ferrier IN, Stanton BB, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246–251. doi: 10.1192/bjp.175.3.246. [DOI] [PubMed] [Google Scholar]
- 27.Ferrier IN, Thompson JM. Cognitive impairment in bipolar affective disorder: implications for the bipolar diathesis. Br J Psychiatry. 2002;180:293–295. doi: 10.1192/bjp.180.4.293. [DOI] [PubMed] [Google Scholar]
- 28.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- 30.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 32.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glahn DC, Bearden CE, Caetano S, et al. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7:546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 35.Gogtay N, Ordonez A, Herman DH, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 36.Botteron KN, Vannier MW, Geller B, Todd RD, Lee BC. Preliminary study of magnetic resonance imaging characteristics in 8- to 16-year-olds with mania. J Am Acad Child Adolesc Psychiatry. 1995;34:742–749. doi: 10.1097/00004583-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Berg DH. Working memory and arithmetic calculation in children: the contributory roles of processing speed, short-term memory, and reading. J Exp Child Psychol. 2008;99:288–308. doi: 10.1016/j.jecp.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Baddeley A. The fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baddeley A. The concept of episodic memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1345–1350. doi: 10.1098/rstb.2001.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaire P, Abdi H, Fayol M. The role of working memory resources in simple cognitive arithmetic. Eur J Cogn Psychol. 1996;8:73–104. [Google Scholar]
- 41.Logie R, Baddeley A. Cognitive processes in counting. J Exp Psychol. 1987;13:310–326. [Google Scholar]
- 42.Salthouse TA, Kail R. Memory Development Throughout the Life Span: The Role of Processing Rate. New York: Academic Press; 1983. [Google Scholar]
- 43.Fuchs LS, Fuchs D, Hamlet CL, Powell SR, Capizzi AM, Seethaler PM. The effects of computer-assisted instruction on number combination skill in at-risk first graders. J Learn Disabil. 2006;39:467–475. doi: 10.1177/00222194060390050701. [DOI] [PubMed] [Google Scholar]
- 44.Sohlberg MM, Mateer CA. Attention Process Training (APT) Puyallup: Association for Neuropsychological Research and Development; 1986. [Google Scholar]
- 45.Atre-Vaidya N, Taylor MA, Seidenberg M, Reed R, Perrine A, Glick-Oberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:120–126. [PubMed] [Google Scholar]
- 46.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 47.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychological impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 48.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]