Figure. 5. MNNG-induced ING2 localization and ING2/p73α complex formation.
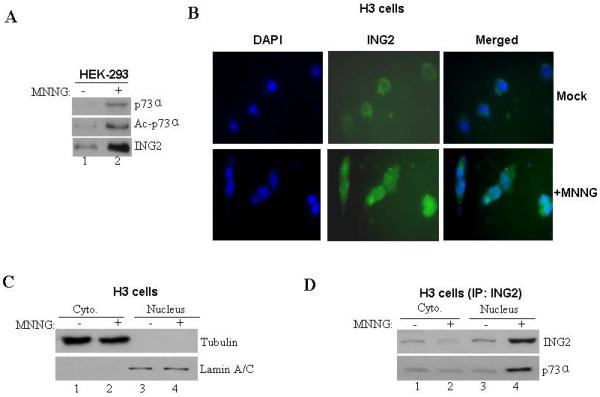
A. Mock- (DMSO) and MNNG- (10 μM; 1h)-treated HEK-293 cells were collected 6 h later. Lysates were formed, adjusted for equal protein content and incubated with anti-ING2 antibody and Protein A Sepharose. The immune-complexes were resolved on a 4-12% gradient SDS-PAGE and analyzed by immunoblotting with anti-73α antibody. The membrane was stripped (described in methods) and re-probed with anti-acetyl lysine antibody. An aliquot of the lysate was also probed with anti-ING2 antibody. B. Mock- and MNNG (10 μM; 1h)-treated HCT116+Ch3 (H3) cells were collected 6 h later cells, fixed and stained with anti-ING2 antibody and subsequently with a FITC-conjugated secondary antibody. Cells were also counterstained with DAPI and visualized under immunofluorescence microscope. C. Mock- and MNNG-treated cells were subjected to hypotonic lysis and nuclei isolated by sucrose cushion centrifugation. Following this, nuclei were lysed and both cytoplasmic (lanes 1 and 2) and nuclear fractions (lanes 3 and 4) were normalized for equal protein content and immunoblotted against tubulin (top panel) and nuclear protein lamin A/C (bottom panel) antibody to confirm the integrity of fractionation. D. Lysates of both cytoplasmic and nuclear fractions from mock- and MNNG-treated cells were used for immunoprecipitation reactions by incubation with anti-p73α antibody. Subsequently, immune-complexes were resolved and immunoblotted with anti-ING2 (top panel) and anti-p73α (bottom panel) antibody.
