Table 3.
Conversion of aryl triflates and nonaflates to nitro aromatics.a
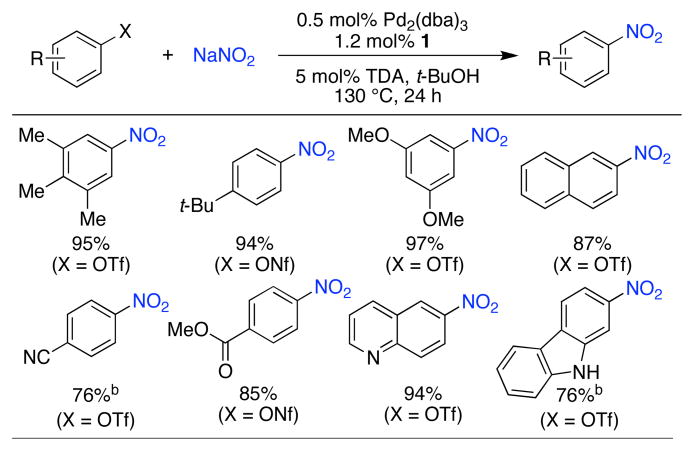 |
ArX (1 mmol), NaNO2 (2.0 mmol), Pd2(dba)3 (0.5 mol%), 1 (1.2 mol%), t-BuOH (2 mL), 130 °C, 24 h; isolated yields, average of 2 runs.
Pd2(dba)3 (2.5 mol%), 1 (6 mol%).
Conversion of aryl triflates and nonaflates to nitro aromatics.a
 |
ArX (1 mmol), NaNO2 (2.0 mmol), Pd2(dba)3 (0.5 mol%), 1 (1.2 mol%), t-BuOH (2 mL), 130 °C, 24 h; isolated yields, average of 2 runs.
Pd2(dba)3 (2.5 mol%), 1 (6 mol%).