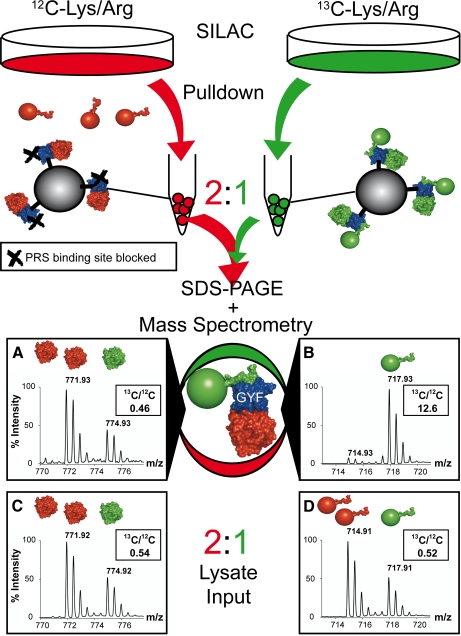
Fig. 1.
Experimental outline for complex analysis. Separate pulldown experiments with GST-GYF fusion proteins and unlabeled (red) or 13C-labeled (green) cell lysates were performed. For the unlabeled cell lysates, the PRS binding site of the GYF domain was blocked either by mutation (W8R/Y33A mutant) or by a competing PRS peptide (2 mm). After washing, GST-GYF and associated complexes from both experiments were mixed in a ratio of 2:1 (unlabeled:labeled), separated by SDS-PAGE, and digested with trypsin. In the subsequent MS/MS analysis, protein complexes associated via the PRS binding site of the GYF domain were 13C-labeled (spectrum B, peptide 150GTIEILSDVQLIK162 of the 60 S ribosomal protein P0), whereas protein complexes bound unspecifically via the U5-15K site to the GYF domain were present in a 2:1 ratio (unlabeled:labeled; spectrum A, peptide 49GVDEVTIVNILTNR62 of annexin A2) and therefore were recognized as unlabeled in the later data analysis. Spectra C and D represent the peptides in spectra A and B, respectively, detected in a 2:1 (unlabeled:labeled) mixture of the input lysates for the pulldown experiment.