Abstract
Purpose
To determine whether the polymorphisms of calcitonin receptor-like receptor gene (CALCRL) are associated with primary angle closure glaucoma (PACG) in a southern Chinese population.
Methods
A total of 207 individuals with acute and chronic PACG and 205 ethnically matched controls were recruited in the current study. A tag-single nucleotide polymorphism approach was used to investigate this gene. Alleles were determined by PCR restriction fragment length polymorphism.
Results
The results show a nominal association between rs1157699 and acute PACG (uncorrected p=0.024 and 0.028 for alleles and genotypes, respectively). Haplotype Trs840617Crs6759535Trs1157699 frequency is significantly higher in acute PACG patients than in controls (corrected p=0.012), whereas haplotype Trs840617Crs6759535Crs1157699 frequency is significantly lower in acute PACG patients compared with controls (corrected p=0.02). However, no significant difference was detected between chronic PACG and CALCRL tag single nucleotide polymorphisms.
Conclusions
The study suggests a possible role of CALCRL in the pathogenesis of acute PACG but not chronic PACG. A replication of this study with a larger sample size as well as on different populations will be helpful in confirming this finding.
Introduction
Glaucoma is the second leading cause of blindness worldwide, and 60 million people will have open angle glaucoma and angle closure glaucoma (ACG) by 2010 [1]. Primary angle closure glaucoma (PACG) is the most common type of glaucoma in the Asian population and is responsible for the vast majority of bilateral glaucoma blindness in China [2]. PACG can be divided into an acute or chronic form. Acute PACG (APACG) has a rapid onset, dramatic symptoms, and severe elevation of intraocular pressure (IOP), while chronic PACG (CPACG) is latent—the presence of peripheral anterior synechiae is gradually formed, resulting in visual field defects. Although their manifestations are different, APACG and CPACG share similar anatomical characteristics and both are caused by factors that either pull or push the iris up into the angle, physically blocking the drainage of aqueous humor and raising IOP [3].
Predisposing factors for PACG are mainly related to the geometry of the anterior chamber, such as a shallow anterior chamber, narrow angle, lens position, lens thickness, and short axial length [4]. Other risk factors include age, female sex, and family history [5]. Lowe [6] suggested that the action of a large number of grouped or independently inherited genes results in anterior chamber shallowness. However only 10% of people with anatomically narrow angles develop PACG [7]. Therefore other factors may alter the degree of pupillary block, thus triggering a PACG attack. Because the pathogenesis of PACG is complex, there has been no single or combination of parameters that is sensitive enough to predict whether eyes with a shallow anterior chamber and “occludable angle” will develop PACG.
Perhaps, it is reasonable to expect that genes involving development and functioning of the anterior segment participate in the geometry of the anterior chamber. A number of lines of evidence have suggested that both genetic and environmental factors contribute to the development of PACG [6]. PACG, as a common complex disease [8], has become an important target for association studies in recent years. PACG is associated with genes related to regulation of axial length and structural remodeling of connective tissues, such as membrane-type frizzled-related protein (MFRP) gene, matrix metalloproteinase 9 (MMP-9) gene, and methylenetetrahydrofolate reductase (MTHFR) gene [9-13]. The biological significance of the associated single nucleotide polymorphisms (SNPs) in the development of PACG is still unknown [9]. However such association has not been replicated in subsequent research [10,11].
Recently, a transgenic mouse model for acute ACG was generated by Lars et al. [14]. Overexpression of calcitonin receptor-like receptor (CALCRL) in the pupillary sphincter muscle resulted in pupillary palsy and acutely and transiently elevated IOP in mice, mimicking the characteristic phenotype of acute PACG in humans. It is unknown whether genetic variations in CALCRL are associated with PACG.
In this study, genetic association analysis was performed between tag SNPs of CALCRL and PACG (both APACG and CPACG) in a southern Chinese population.
Methods
Patient recruitment and assessment
Unrelated PACG patients and control subjects were recruited from the Zhongshan Ophthalmic Center of Sun Yat-sen University (Guangzhou, China). The study subjects consisted of 207 patients with PACG (including 139 females and 68 males , age 50-80) and 205 unaffected controls (including 138 females and 67 males , age 43-95). Written informed consent was obtained from all subjects, and the study had the approval of the Ethics Committees of the Zhongshan Ophthalmic Center and was performed according to the tenets of the Declaration of Helsinki. All control subjects were matched by age and gender with the patients. All study subjects were Han Chinese from southern China.
PACG was defined according to the International Society of Geographical and Epidemiologic Ophthalmology classification by Foster et al. [15]. PACG subjects were further categorized into two groups: APACG and CPACG.
The diagnosis of APACG was based on the following criteria:
(1) the presence of at least two symptoms: eye pain, headache, blurred vision, and vomiting;
(2) the presence of at least three of the following signs: conjunctival congestion, corneal epithelial edema, mid-dilated unreactive pupil, glaucomflecken, and iris atrophy;
(3) 270° or greater of anterior chamber angle closure on gonioscopic examination;
(4) IOP >40 mmHg by Goldmann applanation tonometry.
The diagnosis of CPACG was based on the following criteria:
(1) the presence of glaucomatous optic neuropathy, which was defined as a cup-to-disc ratio ≥0.7 or asymmetry ≥0.2 between the two eyes, neuroretinal rim width reduced to ≤0.1 cup-to-disc ratio, and nerve fiber layer defect;
(2) visual field loss detected with static automated white-on-white threshold perimetry (SITA fast strategy, program 30–2, model 750; Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA) that is consistent with glaucomatous optic nerve damage. This was defined as Glaucoma Hemifield test results outside normal limits and/or an abnormal pattern standard deviation (SD) with p<5% occurrence in the normal population;
(3) a closed angle on indentation gonioscopy. A closed angle was defined as the presence of at least a 180° angle in which the posterior pigmented trabecular meshwork (TM) was not visible on gonioscopy and with evidence of peripheral anterior synechiae in any part of the angle.
Patients were excluded if they had secondary angle closure or a personal history of hypertension, diabetes, or cardiovascular disease.
Control subjects did not have a history of any of the above symptoms and signs. All the control subjects received a complete ocular examination, which included best corrected visual acuity measurements using the logarithm of the minimum angle of resolution 4-m charts, slit-lamp evaluation of the anterior segment, fundus examination, measurement of IOP, axial length measurement (A-scan ultrasonography, Quantel Medical, Clermont-Ferrand, France), and detailed recording of the health and degree of cupping of the optic nerve head. All control subjects had open angles, IOPs of <21 mmHg, normal optic nerve heads with cup-to-disc ratio of ≤0.5, no family history of glaucoma, no ophthalmic diseases except cataract, and no personal history of hypertension, diabetes, or cardiovascular diseases.
DNA Preparation
DNA samples of PACG patients and controls were prepared from leukocytes of peripheral venous blood using the phenol-chloroform extracted method and stored at –80 °C until use.
Tag Single Nucleotide Polymorphism Selection and Genotyping
The Tagger program in Haploview 4.1 was used for selecting tag SNPs. In order to cover most of the genetic variability of the CALCRL gene, tag SNPs were chosen using pair-wise tagging, Hardy–Weinberg equilibrium p value cutoff 0.05, r2 cutoff >0.8, minimum minor allele frequency >0.1, and population CHB (Han Chinese in Beijing, China)+JPT (Japanese in Tokyo, Japan). Finally we chose seven tag SNPs suggested by the Tagger program, which captured 87.7% of common CALCRL SNPs in the Hapmap database. These proposed tag SNPs were: rs7591567 [C/T] at intron 1, rs3821181 [C/G] at intron 1, rs9288141 [A/G] at intron 1, rs1157699 [C/T] at intron 1, rs840617 [A/T] at intron 8, rs6759535 [C/T] at intron 8, and rs3771073 [C/G] at intron 1. The latter two tag SNPs were used in exchange for rs3771081 (D'=1, r2=1.0) and rs12104503 (D'=1, r2=0.905), respectively.
All seven tag SNPs were genotyped by PCR restriction fragment length polymorphism (PCR-RFLP). The primers of the seven sites were designed using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA). The details of the primers and enzymes used for PCR-RFLP genotyping are presented in Table 1.
Table 1. Primer pairs and enzymes used for CALCRL tag snp genotyping.
| dbSNP ID | Forward primer | Reverse primer | Product length (bp) | Tm (°C) | Restriction enzyme |
|---|---|---|---|---|---|
|
rs7591567 |
GTCACGCTGAGGTAGG |
GATTTCATTTGCCACC |
485 |
56 |
BstZ171 |
|
rs3771073 |
CAGAATGTAGCAGGAC |
CTCTAAAGGGATGGTT |
215 |
51 |
Ddel |
|
rs3821181 |
GATACCTAGGGAACCA |
ACAACAGCGAAACATT |
280 |
51 |
AflII |
|
rs9288141 |
CCTGCAAGACAATCCC |
ATCCGACAAGGTGAGC |
381 |
56.6 |
HpaII |
|
rs1157699 |
GATGAAAGCCTGAGAA |
ACCTGCCTCCATACTC |
165 |
52.4 |
BanII |
|
rs6759535 |
TTTATTGAGTGCCTAC |
AAATGGACCATGTTTA |
381 |
50.3 |
HpaII |
| rs840617 | TCCATTGGCTAAGTCC | CCCTTACCCTTTCCAG | 353 | 54.3 | HpyCH4IV |
The PCR reaction was performed in a thermocycler (Biometra, GoÈttingen, Germany) under the following conditions: an initial denaturation at 95 °C for 5 min followed by 33 to 35 cycles of 95 °C for 30 s, proper annealing temperature for 30 s, and 72 °C for 30 s. The final extension cycle of 72 °C was for 5 min. The PCR products were then digested by the proper restriction enzymes (New England Biolabs, Inc., Beijing, China). Digestion products were loaded onto an 8% (49:1) polyacrylamide/0.5× Tris/Borate/EDTA gel and resolved at 35 W for about 1.5 h. The images were recorded digitally.
Statistical Analysis
Statistical analysis was performed with SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL). The Hardy–Weinberg equilibrium was tested by the χ2 test. We evaluated the frequency of genotypes and alleles in this study using the χ2 test or Fisher’s exact test (p values <0.05 were considered significant). The haplotype frequency and linkage disequilibrium of the SNPs were estimated with Haploview 4.1. A haplotype frequency <0.03 was not studied further. All the data were corrected by Bonferroni correction.
Results
There was no difference between the control group and the patients with PACG in gender and age, as shown in Table 2. Seven tag SNPs in CALCRL were determined in patients and controls. The polymorphism at rs3821181 was not detected in all 412 subjects (C/C genotype in all subjects), so it was excluded in the following comparisons. The other six SNPs were in Hardy–Weinberg equilibrium in APACG and CPACG, as well as in controls (p>0.05). The haplotype block structure, together with D’ values and the position of the studied tag SNPs of CALCRL, are shown in Figure 1.
Table 2. Demographic features of PACG and control subjects.
| Demograhphic features | Control subjects (n=205) | APACG subjects (n=109) | p value | CPACG subjects (n=98) | p value | |
|---|---|---|---|---|---|---|
| Gender |
Female |
138 (67%) |
75 (68.8%) |
0.788 |
64 (65.3%) |
0.728 |
| |
Male |
67 (33%) |
34 (31.2%) |
|
34 (34.7%) |
|
| Age |
Mean±SD |
67.12±7.89 |
65.91±8.66 |
0.654 |
65.59±7.16 |
0.677 |
| Range | 50-80 | 45-95 | 43-85 | |||
Figure 1.
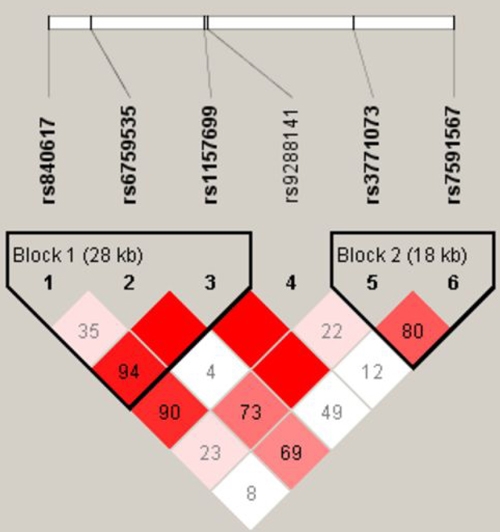
The haplotype block structure and the position of the studied tag single nucleotide polymorphisms of CALCRL. The relative physical position of each tag single nucleotide polymorphism (SNP) is given together with the rs number in the upper part of the diagram. Pair-wise SNP D’ values (multiply by 100) of linkage are shown together with two identified haplotype blocks. The bright red rectangles represent D’=100 (multiply by 100).
Single marker analysis
Allele and genotype frequency comparisons between APACG patients and controls demonstrated nominally significant differences with p values of 0.024 and 0.028, respectively, for the C/T polymorphism at intron 1 (rs1157699), but this significance was lost after Bonferroni correction (corrected p value 0.144 and 0.168, respectively). No significant difference in the remaining five SNPs tested was observed between APACG patients and controls in the distribution of alleles and genotypes (Table 3 and Table 4), and none of the six SNPs were significantly associated with CPACG (Table 3 and Table 4).
Table 3. Frequency of genotypes of six CALCRL snps in PACG patients and controls.
| dbSNP ID | Genotype | Control n (%) | APACG n (%) | p Value | CPACG n (%) | p Value |
|---|---|---|---|---|---|---|
|
rs7591567 |
CC |
5 (2.4%) |
3 (2.8%) |
0.779 |
2 (2%) |
0.368 |
| |
CT |
53 (25.9%) |
32 (29.4%) |
|
33 (33.7%) |
|
| |
TT |
147 (71.7%) |
74 (67.9) |
|
63 (64.3%) |
|
|
rs3771073 |
CC |
4 (2%) |
2 (1.8%) |
0.343 |
1 (1%) |
0.509 |
| |
CG |
57 (27.8%) |
39 (35.8%) |
|
33 (33.7%) |
|
| |
GG |
144 (70.2%) |
68 (62.4%) |
|
64 (65.3%) |
|
|
rs9288141 |
AA |
128 (62.4%) |
68 (62.4%) |
0.816 |
61 (62.2%) |
0.951 |
| |
AG |
72 (35.1%) |
37 (33.9%) |
|
34 (34.7%) |
|
| |
GG |
5 (2.4%) |
4 (3.7%) |
|
3 (3.1%) |
|
|
rs1157699 |
CC |
138 (67.3%) |
57 (52.3%) |
0.028 |
60 (61.2%) |
0.415 |
| |
CT |
60 (29.3%) |
48 (44%) |
|
32 (32.7%) |
|
| |
TT |
7 (3.4%) |
4 (3.7%) |
|
6 (6.1%) |
|
|
rs6759535 |
CC |
95 (46.3%) |
56 (51.4%) |
0.469 |
50 (51%) |
0.742 |
| |
CT |
97 (47.3%) |
44 (40.4%) |
|
42 (42.9%) |
|
| |
TT |
13 (6.3%) |
9 (8.3%) |
|
6 (6.1%) |
|
|
rs840617 |
AA |
18 (8.8%) |
12 (11%) |
0.494 |
5 (5.1%) |
0.488 |
| |
AT |
79 (38.5%) |
35 (32.1%) |
|
37 (37.8%) |
|
| TT | 108 (52.7%) | 62 (56.9%) | 56 (57.1%) |
Table 4. Frequency of alleles of six CALCRL snps in PACG patients and controls.
| dbSNP ID | Allele | Control n (%) | APACG n (%) | p value |
|---|---|---|---|---|
| APACG, n(%) | ||||
|
rs7591567 |
C |
63 (15.4%) |
38 (17.4%) |
0.502 |
| |
T |
347 (84.6%) |
180 (82.6%) |
|
|
rs3771073 |
C |
65 (15.9%) |
43 (19.7%) |
0.221 |
| |
G |
345 (84.1%) |
175 (80.3%) |
|
|
rs9288141 |
A |
328 (80%) |
173 (79.4%) |
0.849 |
| |
G |
82 (20%) |
45 (20.6%) |
|
|
rs1157699 |
C |
336 (82%) |
162 (75.2%) |
0.024 |
| |
T |
74 (18%) |
56 (24.8%) |
|
|
rs6759535 |
C |
287 (70%) |
156 (71.6%) |
0.683 |
| |
T |
123 (30%) |
62 (28.4%) |
|
|
rs840617 |
A |
115 (28%) |
59 (27.1%) |
0.792 |
| |
T |
295 (72%) |
159 (72.9%) |
|
| CPACG, n(%) | ||||
|
rs7591567 |
C |
63 (15.4%) |
37 (18.9%) |
0.276 |
| |
T |
347 (84.6%) |
159 (81.1%) |
|
|
rs3771073 |
C |
65 (15.9%) |
35 (17.9%) |
0.534 |
| |
G |
345 (84.1%) |
161 (82.1%) |
|
|
rs9288141 |
A |
328 (80%) |
156 (79.6%) |
0.910 |
| |
G |
82 (20%) |
40 (20.4%) |
|
|
rs1157699 |
C |
336 (82%) |
152 (77.6%) |
0.201 |
| |
T |
74 (18%) |
44 (22.4%) |
|
|
rs6759535 |
C |
287 (70%) |
142 (72.4%) |
0.535 |
| |
T |
123 (30%) |
54 (27.6%) |
|
|
rs840617 |
A |
115 (28%) |
47 (24%) |
0.289 |
| T | 295 (72%) | 149 (76%) | ||
Haplotype analysis
Pair-wise linkage disequilibrium between the six tag SNPs is shown in Figure 1. We identified two haplotype blocks. In block 1 the frequency of the Trs840617Crs6759535Trs1157699 haplotype was significantly higher in APACG patients than controls (18.2% versus 9.0%, respectively p=0.0015, Bonferroni corrected p=0.012; Table 5), and the frequency of haplotype Trs840617Crs6759535Crs1157699 was significantly lower in APACG patients compared with controls (31.9% versus 42.4% respectively, p=0.0028, Bonferroni corrected p=0.02; Table 5). No association between other haplotypes and CPACG was found (data not shown).
Table 5. Haplotype frequencies in APACG patients and controls.
| Haplotype block | Haplotype | APACG (frequence) | Control (frequence) | Fisher’s p | Corrected p | Odds ratio (95% CI) |
|---|---|---|---|---|---|---|
| block 1 |
ACC |
35.04 (0.161) |
59.35 (0.145) |
0.7395 |
NS |
1.08 (0.69-1.70) |
| |
ACT |
11.79 (0.054) |
16.71 (0.041) |
0.5117 |
NS |
1.29 (0.60-2.77) |
| |
ATC |
9.73 (0.045) |
26.85 (0.065) |
0.2398 |
NS |
0.64 (0.30-1.36) |
| |
TCC |
69.44 (0.319) |
174.03 (0.424) |
0.0028 |
0.02 |
0.59 (0.42-0.83) |
| |
TCT |
39.74 (0.182) |
36.92 (0.090) |
0.0015 |
0.012 |
2.16 (1.33-3.50) |
| |
TTC |
49.80 (0.228) |
75.78 (0.185) |
0.2881 |
NS |
1.25 (0.83-1.86) |
| block 2 |
CC |
28.25 (0.130) |
39.51 (0.096) |
0.2014 |
NS |
1.40 (0.84-2.33) |
| |
CT |
14.75 (0.068) |
25.49 (0.062) |
0.7894 |
NS |
1.10 (0.56-2.13) |
| |
GC |
9.75 (0.045) |
23.49 (0.057) |
0.5028 |
NS |
0.77 (0.36-1.66) |
| GT | 165.25 (0.758) | 321.51 (0.784) | 0.4552 | NS | 0.86 (0.58-1.27) |
95% CI=95% confidence interval; NS=not significant.
Discussion
Adrenomedullin (AM), a 52-amino acid smooth muscle-relaxing polypeptide, is produced in several tissues, including the eye [16]. In human eyes, AM has been identified in the aqueous humor that is produced by the iris ciliary body [17]. Studies in several mammalian species, including humans, have identified the pupillary sphincter as a target of ocular AM [18], and AM receptors linked to cAMP production have been identified [19]. The AM receptor is a complex molecule that consists of CALCRL and receptor activity–modifying protein 2 (RAMP2). CALCRL is a G protein-coupled receptor with seven transmembrane domains. The mechanism for its activation is unique; CALCRL is transported from endoplasmic reticulum to the cell membrane by RAMP2 where it is core glycosylated to become a receptor for AM [20]. The gene encoding CALCRL contains 15 exons interrupted by 14 introns, including one that spans more than 60 kilobases. Exons 1–3 constitute the noncoding region; exons 4 through 15 are coding elements, of which exons 8 to 14 encode seven transmembrane domains [21].
Previous animal studies have demonstrated that the AM gene is associated with abnormalities of vascular function or hypertension [22,23]. However mice that overexpressed CALCRL in smooth muscle-containing tissues had increased CALCRL/RAMP2 (AM receptors) in the pupillary sphincter muscle, resulting in enhanced AM-induced sphincter muscle relaxation. Importantly, certain transgenic mice had acutely and transiently elevated IOP between 1 and 3 months of age before chronically elevated IOP became evident. This indicates that overexpression of CALCRL can lead to increased sensitivity of the sphincter muscle to endogenous AM, thus causing chronic relaxation of the sphincter muscle and, as a consequence, obstruction of the aqueous outflow system.
In humans, PACG is characterized by reduced anterior chamber depth and increased lens thickness. In structurally predisposed eyes, drug-enhanced dilation of the pupil in dim light can provoke PACG [24,25]. Interestingly, dark-adapted transgenic mice exhibited impaired pupillary constriction in response to light exposure. However, topical administration of the AM antagonist AM-(20–50) normalized the pupillary response to light stimulation in the mice, suggesting an AM-mediated functional defect of the sphincter muscle. Taken together we expected that the degree of expression of CALCRL and the receptor activity of its product would affect the occurrence or development of angle closure glaucoma.
To our knowledge, this has been the first study to date investigating relationships between SNPs in CALCRL and PACG in a southern Chinese population. The results show rs1157699 is nominally associated with APACG with a marginal significance (uncorrected p value 0.024 and 0.028 observed in allele and genotype analyses, respectively, of this site). Moreover, our study suggests that the haplotype Trs840617Crs6759535Trs1157699 is positively associated with APACG and that the haplotype Trs840617Crs6759535Crs1157699 might be a protective haplotype for APACG. However we did not find any evidence of association between common variants of CALCRL and CPACG.
In single-marker analysis, we observed that the Trs1157699 allele is significantly more common in APACG patients than controls (uncorrected p=0.02), indicating a possible protective role of the Crs1157699 allele. Although rs1157699, which locates in the first intron of CALCRL, is not a functional SNP, it could have other effects, such as influencing splicing or regulatory processes by affecting the binding of transcription factors to the gene. Furthermore, as a tag SNP, the polymorphism is representative of many other variants, which could be regulatory in the function of the receptor. Also, it may be in linkage disequilibrium with functional variants.
With regard to the haplotype analysis, we found two haplotypes Trs840617Crs6759535Trs1157699 and Trs840617Crs6759535Crs1157699 that are associated with APACG. The former was found to be significantly higher in APACG patients, while the latter was found to be lower in APACG patients. The haplotype association with APACG could be a result of the presence of the Trs1157699 allele, providing significance to the implicated block. This explanation is supported by the fact that the allele frequencies of the other two tag SNPs constructing this haplotype are similar in the investigated populations. Furthermore, the haplotype association is statistically significant after the Bonferroni correction.
To our knowledge, this is the first molecular epidemiology study suggesting a possible association between CALCRL polymorphisms and APACG risk. Despite the significance of SNP rs1157699 not withstanding correction for multiple testing, the results of our exploratory analysis warrant further studies.
The lack of association between the tag SNPs and CPACG suggests that CALCRL is not a significant risk factor for its development. This may be because the mechanism causing CPACG is much more complicated than APACG [26]. In APACG attack, sphincter muscle palsy occurs and the pupil dilates, resulting in pupillary block. With CPACG, multiple mechanisms are involved, for example, closer location of iris root insertion to the angle and thicker and fleshier peripheral irises and anteriorly positioned ciliary bodies. Based on the above reasons, CPACG might be genetically different from APACG.
In conclusion, this study shows a possible association between CALCRL and APACG, and CALCRL may not play a major role in the etiology of CPACG. Additional replication studies are necessary to confirm such association.
Acknowledgments
We thank the grant support from the Guangdong Science and Technology Program (2008B030301334, to X.L.) and the National 863 Plan of China (04AA104092 to X.G.).
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip JL, Foster PJ. Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol. 2006;17:175–80. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 3.Coleman AL. Glaucoma. Lancet. 1999;354:1803–10. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 4.Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin Experiment Ophthalmol. 2000;28:253–8. doi: 10.1046/j.1442-9071.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 5.Amerasinghe N, Aung T. Angle-closure: risk factors, diagnosis and treatment. Prog Brain Res. 2008;173:31–45. doi: 10.1016/S0079-6123(08)01104-7. [DOI] [PubMed] [Google Scholar]
- 6.Lowe RF. Primary angle-closure glaucoma. Inheritance and environment. Br J Ophthalmol. 1972;56:13–20. doi: 10.1136/bjo.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Wu H, Fan Z. Primary angle closure glaucoma in Chinese and Western populations. Chin Med J (Engl) 2002;115:1706–15. [PubMed] [Google Scholar]
- 8.Tombran-Tink J, Karim F, Damji R, Allingham R. Mechanisms of the Glaucomas. Tombran-Tink J, Barnstable CJ, Shields MB, editors. Ophthalmology Research. 3rd edition; New York: Humana Press, 2008. p. 191-203. [Google Scholar]
- 9.Michael S, Qamar R, Akhtar F, Khan WA, Ahmed A. C677T polymorphism in the methylenetetrahydrofolate reductase gene is associated with primary closed angle glaucoma. Mol Vis. 2008;14:661–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang IJ, Chiang TH, Shih YF, Lu SC, Lin LL, Shieh JW, Wang TH, Samples JR, Hung PT. The association of single nucleotide polymorphisms in the MMP-9 genes with susceptibility to acute primary angle closure glaucoma in Taiwanese patients. Mol Vis. 2006;12:1223–32. [PubMed] [Google Scholar]
- 11.Aung T, Yong VH, Lim MC, Venkataraman D, Toh JY, Chew PT, Vithana EN. Lack of association between the rs2664538 polymorphism in the MMP-9 gene and primary angle closure glaucoma in Singaporean subjects. J Glaucoma. 2008;17:257–8. doi: 10.1097/IJG.0b013e31815c3aa5. [DOI] [PubMed] [Google Scholar]
- 12.Aung T, Lim MC, Wong TT, Thalamuthu A, Yong VH, Venkataraman D, Venkataraman A, Vithana EN. Molecular analysis of CHX10 and MFRP in Chinese subjects with primary angle closure glaucoma and short axial length eyes. Mol Vis. 2008;14:1313–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang IJ, Lin S, Chiang TH, Chen ZT, Lin LL, Hung PT, Shih YF. The association of membrane frizzled-related protein (MFRP) gene with acute angle-closure glaucoma--a pilot study. Mol Vis. 2008;14:1673–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Ittner LM, Schwerdtfeger K, Kunz TH, Muff R, Husmann K, Grimm C, Hafezi F, Lang KS, Kurer MO. Götz J, Born W, Fischer JA. Transgenic mice with ocular overexpression of an adrenomedullin receptor reflect human acute angle-closure glaucoma. Clin Sci (Lond) 2008;114:49–58. doi: 10.1042/CS20070163. [DOI] [PubMed] [Google Scholar]
- 15.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udono-Fujimori R, Udono T, Totsune K, Tamai M, Shibahara S, Takahashi K. Adrenomedullin in the eye. Regul Pept. 2003;112:95–101. doi: 10.1016/s0167-0115(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi T, Kawase K, Gu ZB, Kimura M, Okano Y, Kawakami H, Tsuji A, Kitazaway Y. Ocular effects of adrenomedullin. Exp Eye Res. 1999;69:467–74. doi: 10.1006/exer.1999.0737. [DOI] [PubMed] [Google Scholar]
- 18.Uchikawa Y, Okano M, Sawada A, Asada Y, Kobayashi H, Wada A, Nao-j N, Ohkura M, Tanaka N, Yamamoto R. Relaxant effect of adrenomedullin on bovine isolated iris sphincter muscle under resting conditions. Clin Exp Pharmacol Physiol. 2005;32:675–80. doi: 10.1111/j.0305-1870.2005.04249.x. [DOI] [PubMed] [Google Scholar]
- 19.Yousufzai SY, Ali N, Abdel-Latif AA. Effects of adrenomedullin on cyclic AMP formation and on relaxation in iris sphincter smooth muscle. Invest Ophthalmol Vis Sci. 1999;40:3245–53. [PubMed] [Google Scholar]
- 20.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 21.Nakazawa I, Nakajima T, Harada H, Ishigami T, Umemura S, Emi M. Human calcitonin receptor-like receptor for adrenomedullin: genomic structure, eight single-nucleotide polymorphisms, and haplotype analysis. J Hum Genet. 2001;46:132–6. doi: 10.1007/s100380170100. [DOI] [PubMed] [Google Scholar]
- 22.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98:615–9. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–71. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 24.Reuser T, Flanagan DW, Borland C, Bannerjee DK. Acute angle closure glaucoma occurring after nebulized bronchodilator treatment with ipratropium bromide and salbutamol. J R Soc Med. 1992;85:499–500. doi: 10.1177/014107689208500828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein NE, Goldbloom DS. Oral imipramine and acute angle-closure glaucoma. Arch Ophthalmol. 1995;113:698. doi: 10.1001/archopht.1995.01100060020008. [DOI] [PubMed] [Google Scholar]
- 26.Shukla S, Damji KF, Harasymowycz P, Chialant D, Kent JS, Chevrier R, Buhrmann R, Marshall D, Pan Y, Hodge W. Clinical features distinguishing angle closure from pseudoplateau versus plateau iris. Br J Ophthalmol. 2008;92:340–4. doi: 10.1136/bjo.2007.114876. [DOI] [PubMed] [Google Scholar]


