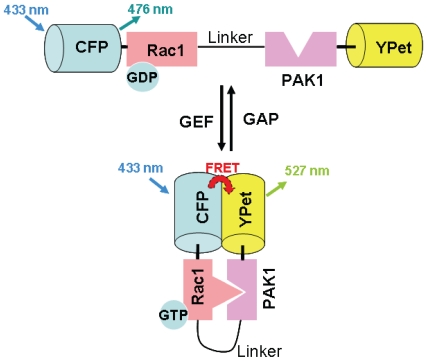
Figure 1. Quantification of Rac activity in a living cell by FRET.
Top, the Rac GTPase (Rac1) and its substrate PAK1 are connected by a flexible linker. Rac1 is associated with Cyan fluorescent protein (CFP) and its substrate PAK1 (p21-activated kinase 1) (a serine-threonine kinase activated by Rac GTPase) with YPet (a variant of yellow fluorescent protein). When Rac is not activated, excitation at 433 nm yields an emission of 476 nm via CFP, no FRET occurs. Bottom, when Rac is activated by GEF (guanine nucleotide exchange factor) that stimulates GTPases by catalyzing the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP), Rac1 proceeds with conformational changes so that it binds specifically with its substrate PAK1. The binding of Rac1 with PAK1 leads to close association of CFP with YPet. Excitation of CFP at 433 nm now yields an emission of 527 nm via YPet, FRET occurs. In the presence of GAP (guanosine triphosphatase (GTPase)-activating protein) that hydrolyses GTP to GDP, Rac1 dissociates from PAK1, the Rac biosensor returns to inactivated and extended form. (Modified from [6]).