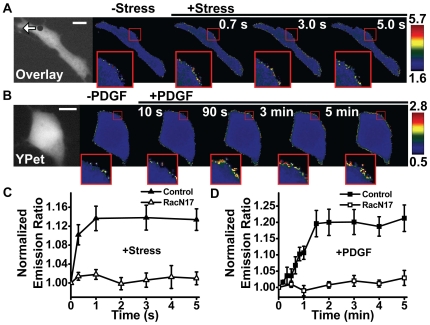
Figure 2. Rapid Rac activation in response to a local mechanical stress.
(A) A 4.5-µm RGD-coated ferromagnetic bead was attached to the apical surface of the cell (black dot is the bead) for 15 min to allow integrin clustering and formation of focal adhesions around the bead. The bead was magnetized horizontally and subjected to a vertical magnetic field (step function) which applies a mechanical rotational stress (apparent average stress = 17.5 Pa) to the cell. A genetically encoded, CFP-YPet cytosolic Rac reporter was transfected into the smooth muscle cells following published procedures. The cytosolic Rac reporter was uniformly distributed in the cytoplasm (YPet fluorescence; white arrow indicates bead movement direction). The stress application induced rapid changes (<0.3 s) in FRET of the Rac reporter at discrete, distant sites at the cell periphery (the focal plane is ∼1 µm above cell base), indicating rapid Rac activation (see Insets). Images are scaled and regions of large FRET changes (strong Rac activity) are shown in red/yellow. Scale bar = 10 µm. (B) Time-lapse images of Rac activation at the cell periphery after addition of PDGF (10 ng/ml) shows that activation of Rac in a representative cell by soluble factor PDGF is slow. Note that significant Rac activation occurred only at 0.5–1 min after PDGF treatment. Insets are enlarged areas showing Rac activation at the cell periphery. Scale bar = 10 µm. (C) Normalized emission ratio of FRET as a function of stress application duration for the Rac biosensor (control) and its mutant form(RacN17) (Control, n = 6 cells; RacN17, n = 3 cells; mean +/− s.e.). A representative cell was shown in A with the Rac biosensor. (D) Normalized YPet/CFP emission ratio time courses of the Rac biosensor (Control) and the mutant Rac biosensor (RacN17) in response to PDGF treatment. (Control, n = 4 cells; RacN17, n = 3 cells; mean +/− s.e.).