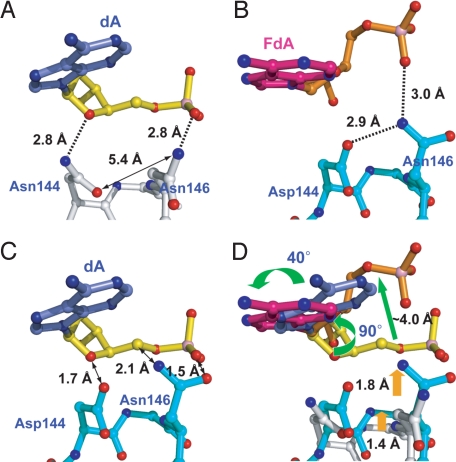
Fig. 4.
Effect of Asn versus Asp at position-144 on the interactions with Asn 146 and with DNA. (A) LRC structure, with the mutated residue Asn-144; (B) FLRC structure, with the wild-type active site Asp-144. Note the differences in the conformation of the protein with Asn-144/Asn-146 (A) versus Asp-144/Asn-146 (B); the former is referred to in the text as the alternative conformation. (C) Heavy atom superposition of the Asp-144/Asn-146-containing loop from the FLRC and substrate adenosine from the LRC. Note the steric clash indicated by a double-headed arrow. (D) Superposition of the substrate adenosine and the loop from both structures. Orange arrows indicate major positional shifts in the loop, and green arrows major shifts in DNA, upon conversion from the LRC to the FLRC.