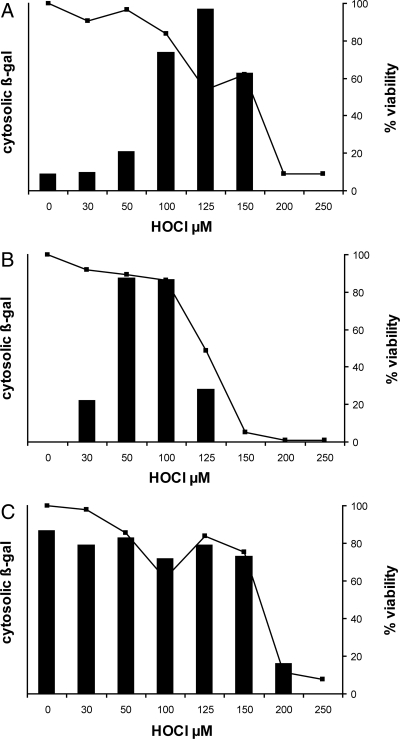
Fig. 5.
HOCl effects on protein translocation from cytoplasm to cell envelope. (A) E. coli strain EC626 (MC4100 Φ(lamB-lacZ) Hyb 42–1), which synthesizes a fusion protein that is translocated through the secA pathway, was exposed to HOCl at the indicated concentrations and then induced to synthesize its normally translocated β-gal fusion protein as described in Materials and Methods. Failure to translocate the protein resulted in accumulation of cytoplasmic β-galactosidase activity (bars). Bacterial viability at each HOCl concentration is shown as a line graph (squares, right y axis). (B) Oxidation conditions as for (A) except that the E. coli strain was EC628 (MC4100 Φ(H*lamB‘-’lacZ), H*lamB [diploid]), which synthesizes a fusion protein that is translocated through the signal recognition particle pathway. Induction and β-gal assay conditions were modified to detect the lower overall fusion protein levels in this strain (see Materials and Methods). (C) Oxidation conditions as for (A) except that the E. coli strain was EC627 (MC4100 Φ(lamBΔ60-lacZ) Hyb 42–1), which synthesizes a fusion protein that is predominantly cytosolic (not translocated) under all conditions. This strain serves as an indicator of bacterial capacity, upon HOCl oxidation, to sense an inducing stimulus and respond with new protein synthesis. Each panel reflects a representative experiment from at least three replicates. Maximal ONPG hydrolysis in (A–C) (A420-1.75*A550; see Materials and Methods), was 0.097, 0.210, and 0.083 units, respectively.