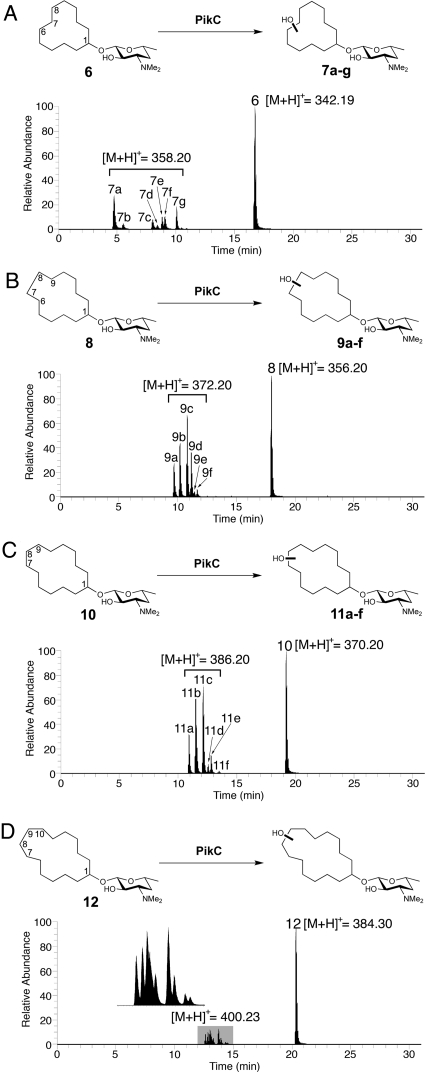
Fig. 3.
LC-MS analysis of PikCD50N-RhFRED catalyzed reactions using different cyclized carbolides as substrates. (Ion count chromatograms are shown.) (A) Desosaminyl cyclododecane (structure 6) reaction; structures 7b and 7f correspond to two diastereomers generated by C7 hydroxylation; structures 7a, 7c, 7e, and 7g correspond to four diastereomers generated by C6/C8 hydroxylation. (B) Desosaminyl cyclotridecane (structure 8) reaction; structures 9a, 9d, and 9f correspond to diastereomers arising from C6/C9 hydroxylation; structures 9b and 9c correspond to diastereomers originated from C7/C8 or C6/C9 hydroxylation. The number of products that peak 9c contains is undetermined because of product overlap. (C) Desosaminyl cyclotetradecane (structure 10) reaction. (D) Desosaminyl cyclopentadecane (structure 12) reaction. The details of product assignment for structures 6 and 8 based on correlation with synthesized authentic standards regarding retention time and coinjection confirmation are shown in the SI Appendix.