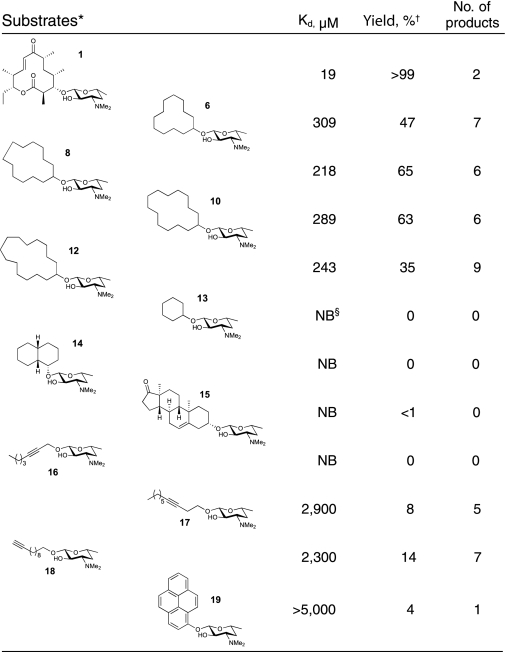
Table 1.
The activity of PikCD50N-RhFRED toward various substrates
*Racemic cis-decahydro-1-naphthol was used in preparation of structure 14, leading to the structure shown and its diastereomer. The mixture of diastereomers was subjected to the PikC oxidation reaction.
†The yield shown is based on product formation calculated with AUCtotal products/(AUCtotal products + AUCunreacted substrate) by assuming ionization efficiency of substrate and various hydroxylated products are the same, because the ionization site of this series of desosaminyl derivatives should be the dimethylamino group. The calculated yield is fairly close (difference < ±3%) to the substrate conversion ratio calculated by 1 − AUCunreacted substrate/AUCtotal substrate (AUCtotal substrate is derived from the control reaction using boiled enzyme under identical conditions), indicating the assumption is correct. (AUC: area under curve of the ion count chromatograms, as shown in Fig. 3).
‡The number of products refers to the number of distinct peaks seen in the liquid chromatography-mass spectrometry (LC-MS). Comparison to synthetic standards illustrated a few cases where regioisomers or diastereomers were not distinguished by LC-MS, so the number provided is a lower limit. See SI Appendix for details.
§NB, no binding.