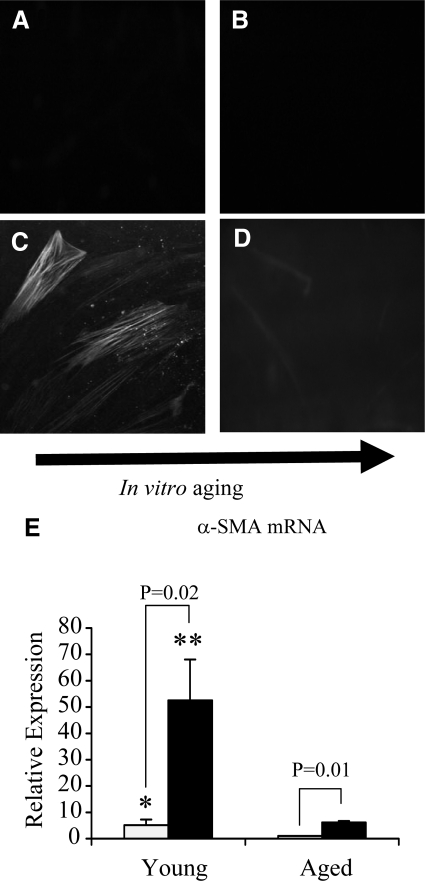
Figure 1.
Immunohistochemical analysis of TGF-β1-dependent α-SMA expression in aging fibroblasts. Monolayers of patient-matched young (A and C) and aged (B and D) dermal fibroblasts were growth-arrested in serum-free medium for 48 hours. The medium was then replaced with either serum-free medium alone (A and B) or serum-free medium containing 10 ng/ml TGF-β1 (C and D) for 72 hours. The cells were then fixed and stained for α-SMA, as described in Materials and Methods. The cells were then mounted in Vectashield fluorescent mountant and viewed under UV light. All results shown are representative of dermal fibroblasts from three patient donors. Original magnification, ×100. E: In a parallel experiment expression of α-SMA mRNA was quantified by QPCR. Confluent monolayers of patient-matched young and aged dermal fibroblasts were growth-arrested in serum-free medium for 48 hours, before addition of either serum-free medium alone (white bar) or serum-free medium containing 10 ng/ml TGF-β1 (black bar) for 72 hours. Total mRNA was extracted, and α-SMA expression was assessed by RT-QPCR. Ribosomal RNA expression was used as an endogenous control, and gene expression was assessed relative to control (unstimulated) aged fibroblasts. The comparative CT method was used for relative quantification of gene expression, and the results represent the mean ± SE of dermal fibroblasts from nine individual experiments with cells isolated from three patient donors. Statistical analysis was performed by the Student’s t-test: *P < 0.05; **P < 0.01, compared with aged cells.