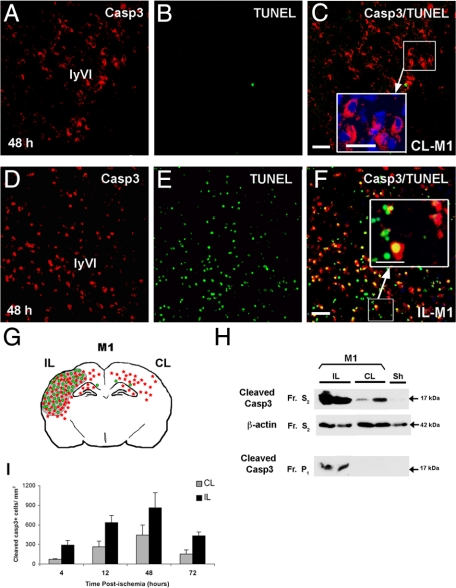
Figure 1.
Neonatal ischemia triggers activation of caspase-3 (casp3) in both hemispheres. Cleaved casp3 immunolabeling (red) and TUNEL assay (DNA fragmentation, green) in sections of brain at the level of the dorsal hippocampus (bregma −3.3 mm) from contralateral (CL-M1, A–C) and ipsilateral (IL-M1, D–F) hemispheres at 48 hours postischemia (model M1). Note that active casp3 was cytosolic in the CL cortex (enlarged panel in C), whereas it was nuclear and cytosolic and associated with TUNEL staining in the IL cortex (enlarged panel in F). Scale bar represents 50 and 20 μm in enlarged panels. G: Spatial distribution of cleaved casp3- (red) and TUNEL-positive (green) cells and lesion area (gray) at 48 hours after ischemic injury in P7 rats in both IL and CL hemispheres. H: Representative Western blots probed with anti-active casp3 (17 kDa; Cell Signaling Technology) for protein samples isolated from the cytosolic (S2) and nuclear (P1) fractions of IL and CL cortex from ischemic rats sacrificed 48 hours after reperfusion and from sham (Sh) brain. The cytosolic marker β-actin was used as protein loading control. Note that p17 was present in the cytosolic fractions in both IL and CL tissues. In contrast, p17 was only present in the nuclear fraction for IL tissues; Fr, fraction. I: Quantification of casp3-positive cells at 4, 12, 48, and 72 hours postischemia in both IL and CL cortex.