Abstract
Objectives. We examined whether a home-based educational and environmental intervention delivered by lay health educators would improve asthma symptom control in inner-city children with asthma.
Methods. Children 2 to 16 years of age with diagnosed asthma and at least 1 asthma-related hospitalization or 2 emergency visits in the prior year were randomly assigned into 2 groups (immediate and delayed intervention) in a crossover study. Each group participated in the active phase (intervention) and the inactive phase. Outcomes included asthma symptoms, albuterol use, emergency department visits, hospitalizations, and trigger reduction.
Results. A total of 264 primarily Black (94%) children were enrolled. The mean number of emergency visits decreased by 30% and inpatient visits decreased by 53% (P < .001) after the intervention. Reductions were seen in pests, presence of carpets in bedrooms, and dust. Nighttime wheezing was significantly reduced after the intervention in both groups (P < .001).
Conclusions. Lay health educators effectively reduced asthma triggers and increased caregiver asthma knowledge, which resulted in reduced emergency department visits, hospitalizations, and asthma symptoms. The relationships formed between the caregivers and the lay health educators appeared to positively impact asthma outcomes in this disadvantaged population.
Asthma prevalence rates and morbidity indices are historically high, having doubled from the 1980s to the 1990s.1 With annual estimates of 12.3 million physician office visits and 1.8 million visits to emergency departments for asthma, the disease exerts a large cost and resource burden on the United States health care system.2 Asthma places a huge burden on families regarding medical care, psychosocial stressors, and daily living. This burden is magnified in populations who are poor, African American, Hispanic, or disadvantaged.3 The 2- to 3-fold higher rate of emergency department visits by African Americans and Hispanics reflects the disproportionate burden.2 The causes of these disparities are many, including disparities in appropriate asthma care, environmental exposures, and other psychosocial issues.3
Asthma management includes medical therapy as well as allergen avoidance.4 The current national asthma guidelines recommend focus on symptom control as a function of appropriate asthma management. Symptoms include wheezing, coughing, and chest tightness in the day and night. Reduction of these symptoms is considered improved control, as is less use of quick-relief medicines. Environmental asthma allergen mitigation has been demonstrated to reduce inpatient hospitalizations and emergency department visits and to reduce some asthma symptoms.4 The cost of the various strategies used to reduce exposure to these aeroallergens, however, are potentially prohibitive for disadvantaged populations.5
The Community Asthma Prevention Program of The Children's Hospital of Philadelphia partnered with The Children's Services Incorporated and the University of Pennsylvania Institute of Environmental Health Sciences to design and implement an asthma environmental intervention to address these disparities in a minority inner-city community characterized by overcrowding, older dilapidated housing, and limited resources. The environmental justice issue raised was, how do we empower residents of this community to reduce the asthma burden in their children?
In light of studies demonstrating the importance of asthma symptom control, the partners decided to implement and study a low-cost educational and environmental mitigation intervention as a potential solution for improving symptom control, thereby reducing inpatient and emergency department visits. Previous studies have shown that home environmental education along with mitigation efforts can reduce asthma symptoms.6–8 For example, the National Cooperative Inner City Asthma Study (NCICAS),9 a multisite randomized controlled study, included tailored environmental interventions and asthma counseling by a master's-prepared social worker. All participants received dust and cockroach mitigation, but other environmental interventions were based on the child's skin-testing results. The NCICAS outcomes showed that this intervention method led to reduced asthma symptoms. Given that the focus of our environmental justice intervention was on a similar inner-city population, our intervention targeted the common indoor triggers found in NCICAS–dust, cockroaches, rodents, pets, and tobacco smoke exposures—but tailored the interventions on the basis of general exposure to these triggers instead of on specific allergens found from skin tests.
Our rationale for this more generalized approach was that atopic children with repeated exposures to indoor allergens can eventually develop asthma symptoms from exposure to the perennial allergens.10 Additionally, because most disadvantaged urban children with asthma are managed by primary-care providers rather than specialists, we sought to look at a low-cost approach that would be more easily disseminated among these communities, thereby removing the social barriers that prevent many families from attending specialist visits. Another unique aspect of our study was the use of community health workers or lay health educators to implement the intervention rather than a master's-level trained professional. The use of lay health educators allowed families to be effectively taught and trained at a relatively lower cost by peers who lived in their communities and faced similar social barriers. Previously, we conducted a randomized controlled trial to study this approach in which we followed the participants for 1 year. We found that both control and intervention groups experienced improved outcomes, whereas the placebo group did not.6 In the present study, we looked at the effectiveness of a 6-month-long intervention period to determine whether similar outcomes could be obtained.
We hypothesized that empowering families with education and environmental supplies would lead to sustainable practices in the homes of children with asthma and would reduce asthma morbidity. We describe the impact of a low-cost environmental mitigation program on symptom control, use of short-acting β-agonists, and the number of asthma-related inpatient and emergency department visits in urban, disadvantaged children with asthma.
METHODS
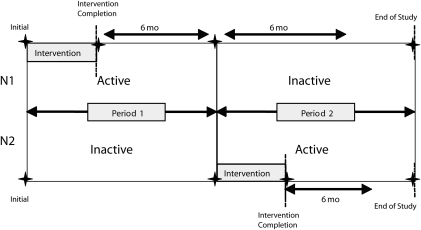
We used a randomized crossover design (Figure 1) to evaluate the changes in participant behavior and asthma morbidity that occurred as a result of the educational and environmental asthma intervention. Study participants were enrolled from February 2002 through February 2005. A secondary aim was to observe whether these changes were maintained once the supports for behavior change (supplies and homes visits) were reduced. The design also allowed for the evaluation and comparison of participants' changes in behavior as the result of the lay health educators' presence before the educational or environmental intervention was implemented. Participants were randomly assigned to either the immediate intervention group to begin with the active intervention phase or the delayed intervention group to begin with the inactive intervention phase lasting 6 months. The active intervention phase, for both groups, consisted of 5 visits to complete the home education and environmental intervention and a 6-month period of follow-up assessments. Written consent was obtained from all caretakers in the study, and written consent or assent was obtained from all children 7 years or older. Previously, a randomized controlled study conducted by the same investigators found that there were similar reductions in clinical outcomes in both the control and intervention groups. With the 6-month crossover design we sought to examine (1) whether the immediate intervention group would have changes at 6 months that would be sustained (a carryover effect), (2) whether the presence of the lay health educator in the delayed intervention group would cause behavior changes in the first 6 months before the intervention, and (3) whether similar outcomes could be accomplished in 6 months compared with 1 year.
FIGURE 1.
Study design of the asthma environmental mitigation intervention.
Note. N1 = immediate intervention group; N2 = delayed intervention group.
Inclusion Criteria
We enrolled children aged 2 to 16 years who had asthma diagnosed by a physician and who were taking a controller medication for treatment. Participants enrolled also had to be residents in 1 of the west or southwest Philadelphia zip codes, where a majority of the households are minorities (69%), and 26% have incomes under the poverty level. Other eligibility criteria included having at least 1 asthma-related inpatient or 2 asthma-related emergency department visits or urgent doctor visits within the year before enrollment. Participants were recruited by their treating physician or by self-referral. Patients were not contacted by research staff unless their medical records showed that they met the eligibility criteria. Self-referrals were usually from partner agencies aware of the eligibility criteria.
Training of Lay Health Educators
Lay health educators implemented the home intervention. Their qualifications included residence in West Philadelphia, at least a high school education with 3 years of experience, and a car available to them for transportation. Two full-time lay health educators were hired and received intensive didactic training about asthma, asthma symptoms and triggers, environmental trigger removal methods, asthma medications, adult learning styles, and proper use of asthma devices. The lay health educators were required to demonstrate their knowledge of this information through practical assessments before they worked independently with families. Training also included sessions about study design, review of data collection tools and data collection processes (including instruction on dust collection methods), confidentiality, privacy, and safety practices. Information regarding community resources was made available. Mock scenarios were held in the final session to assess the educators' knowledge and ability to answer commonly asked questions and concerns. Problems that could be encountered in the home were incorporated into the practicum, which had to be successfully completed before the educators were able to start actual visits. The “buddy-method,” in which the lay health educators were accompanied by a project manager or an experienced lay health educator in the field, was used until the lay health educator was considered competent in all aspects of the intervention. The lay health educators were assigned to specific families and carried a running caseload of 45 participants.
Environmental Mitigation and Education Intervention
After the lay health educators received a family's consent to participate, the family was randomly assigned to the immediate intervention group or the delayed intervention group. In the active phase, the lay health educators conducted home education and environmental interventions over 5 sessions. In general, the participating families were consistently visited by the same lay health educator. Using the “You Can Control Asthma” curriculum (validated and distributed by the Asthma and Allergy Foundation), the educational intervention was designed to teach families asthma pathophysiology, recognition of symptoms, recognition and avoidance of triggers, and appropriate treatment. The environmental intervention targeted dust, pests, pets, and smoke. The lay health educators taught families appropriate avoidance measures for dust, pests, pets, and smoke and assisted families in the implementation of these measures in the child's bedroom. Supplies given to families included mattress and pillow covers, roach bait, mice traps, cleaning aids, shades to replace curtains, tiles to replace carpet, and storage bins to decrease clutter in the child's bedroom. Families were encouraged to make similar changes throughout the home within their own means and were given information on where similar supplies could be purchased if they were interested in doing so.
Follow-up assessments occurred for 24 weeks during the active phase and consisted of biweekly visits to the participants' homes to collect asthma diaries in which the child's daytime and nighttime asthma symptoms and medication use were recorded. During 1 of the visits each month, a bedroom assessment would also be completed to check on the status of the presence of asthma triggers. During the monthly bedroom assessments, the lay health educators would check the child's bedroom for evidence of targeted triggers and the maintained and proper use of the supplies given to the families during the intervention period, such as shades, mattress and pillow covers, and storage bins. The lay health educators' observations were recorded and used for analysis of trigger reduction. The average cost of the supplies was $121 per child.
The inactive phase consisted of only 1 visit each month for 6 months in which both bedroom assessments and asthma diary collections were implemented. Because the inactive phase occurred after the educational and environmental intervention was already completed for the immediate intervention group, this period served as a measure of long-term maintenance for this group. On the other hand, in the delayed intervention group, the inactive phase occurred during the first period, before the intervention, and acted as a measure of comparison with itself before the intervention and as a comparison with the immediate intervention group during their active phase.
Assessments
Baseline assessments of the participant's asthma history, social demographics, and home environment were completed at the initial visit. The initial environmental assessment included parent report, visual assessment, asthma knowledge, and dust sampling from the child's bedroom. Asthma knowledge was measured by using a 16-question multiple-choice test administered by the lay health educator at baseline and at the end of the educational intervention for both the immediate and delayed intervention groups.
Monthly bedroom assessments and self-reported diary information were collected from both groups and were used to accumulate data on asthma triggers present in the home and patient symptom information. Diaries were used to collect data on daytime and nighttime coughing and wheezing, missed school or work days because of asthma, and medications usage. Dust samples for the Aclotest kit (ALO Laboratories, Inc, Columbus, OH) were collected at baseline and then at the completion of the intervention, approximately 12 weeks after the first intervention visit. The Aclotest kit is a simple test used to measure the amount of dust antigen collected by the vacuum cleaner. The results were read as negative, weakly positive, positive, and strongly positive. The lay health educators were given detailed instructions to collect dust from 3 locations in the child's bedroom using the vacuum cleaner with a new filter for each sample. At least 1 of these areas had to be under the bed. At each location, they moved the vacuum in a gridlike fashion over a 1-foot square area, after which the dust sample was collected and sealed.
Statistical Analysis
Patient characteristics, baseline asthma morbidity, and home characteristics were compared between the immediate and delayed intervention groups. For the continuous variable age, a 2-sample t-test was performed. For the number of emergency department (inpatient) visits in the previous year, a Wilcoxon rank-sum test was performed. For categorical variables including gender, race/ethnicity, caretaker education, caretaker employment, housing, and environmental triggers, the χ2 or Fisher exact test was used. The Kruskal-Wallis test was used for the analysis of ordinal nighttime symptom variables.
The Wilcoxon signed-rank test was performed for the paired comparison of asthma knowledge before and after the intervention for both groups. Analysis of this outcome was completed on all participants who possessed both baseline and postintervention data. The Wilcoxon rank-sum test was used for the comparison of the difference in asthma knowledge before and after the intervention between the immediate and delayed intervention groups. Dust antigen was analyzed similarly by using the first test after the initial visit and the first test after intervention completion.
Improvement of asthma triggers (roach, rodent, smoker, furry pet, carpet, and dust due to lack of mattress cover and pillow cover) was defined for the active period of the immediate intervention group as a reduction between the initial visit and 2.5 months after intervention completion and for the inactive period as a reduction between 6 months after intervention completion and 2.5 months later. For the delayed intervention group, improvement was defined as a reduction between the initial visit and 2.5 months later for the inactive period and between the initial visit and 2.5 months after intervention completion for the active period. These measurements of improvement of asthma triggers are binary, and each patient had 2 measurements during inactive and active periods. The χ2 test was performed to compare the proportions of improvement between the immediate and delayed intervention groups in the active and inactive periods separately. In addition, to adjust for the correlation of the repeated measurements within each patient and in consideration of the binary outcome, the generalized estimating equation method for binomial distribution was used to assess the period and treatment effects on these improvements.
To analyze the count data and account for the correlation of the repeated measurements for each patient, the generalized estimating equation method with Poisson distribution was used to model the emergency department visits to examine treatment, treatment group, and age group effects. Inpatient visits were analyzed similarly.
Weekly diaries were used to record asthma symptoms. The number of times per week with coughing or wheezing symptoms or albuterol usage was collected as none, 1–2 days, 2–4 days, and every day. A point value was assigned to each category as follows: none, 0; 1–2 days, 1.5; 2–4 days, 3.5; 4–7 days, 6. This score for each diary period was used for analysis of symptoms and albuterol usage. To adjust for the correlation between the repeated measurements, and considering the continuous nature of these outcome measurements, linear mixed effect models were fitted for the immediate and delayed intervention groups separately to examine the change of symptoms (night cough, night wheeze, and albuterol usage) over time since intervention completion with control for baseline effect and age.
All analyses were performed by using the statistical software package SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 264 children, mean age of 6 years, were enrolled in the study. A total of 144 children were randomly assigned to the immediate intervention group (active phase first), and 120 were randomly assigned to the delayed intervention group (inactive phase first). Seventy-seven percent of the participants completed the project. The dropout rate was higher (P = .033) in the delayed (29%) than in the immediate intervention group (18%).
No significant differences in baseline characteristics were found between the immediate intervention group and the delayed intervention group (Table 1). Most participants were Black, non-Hispanic (94%); 66% were males (n = 174), and 76% of the families in the study lived in a row home. At baseline, 53% of the families had carpet at home, 56% had carpet in the child's bedroom, 47% had roaches, 57% had rodents, 41% had pets, and 50% had smokers present. There was no significant difference in the number of emergency department visits in the year before enrollment in the study between the immediate intervention group (mean = 2.26) and the delayed intervention group (mean = 2.35). This was also true for the number of hospitalizations (mean = 0.85 for the immediate intervention group and 0.95 for the delayed intervention group).
TABLE 1.
Participant Demographic Characteristics, Home Characteristics, and Baseline Asthma Morbidity Characteristics: Philadelphia, PA, 2002–2004
| Characteristic | Immediate Intervention Group (n = 144) | Delayed Intervention Group (n = 120) | P |
| Child's age,a y, mean (SD) | 5.9 (3.3) | 6.2 (3.6) | .44 |
| Boy,b no. (%) | 94 (65) | 80 (67) | .81 |
| Black, non-Hispanic,b no. (%) | 136 (94) | 112 (93) | .74 |
| Caretaker completed high school,b no. (%) | 112 (78) | 97 (81) | .54 |
| Caretaker employed,b no. (%) | 60 (42) | 60 (50) | .18 |
| Housing,b no. (%) | .25 | ||
| Single | 3 (2) | 3 (3) | |
| Row house | 105 (73) | 95 (79) | |
| Apartment | 35 (24) | 19 (16) | |
| Other | 1 (1) | 3 (3) | |
| Environmental triggers,b no. (%) | |||
| Home has carpet | 70 (49) | 70 (58) | .12 |
| Child's bedroom has carpet | 77 (53) | 72 (60) | .29 |
| Mattress in mattress cover | 10 (7) | 9 (8) | .86 |
| Pillow in pillow cover | 6 (4) | 2 (2) | .30 |
| Smoker resides in house | 74 (51) | 58 (48) | .62 |
| Roaches | 70 (49) | 54 (45) | .56 |
| Rodents | 90 (63) | 61 (51) | .06 |
| Furry Pet | 58 (40) | 49 (41) | .93 |
| ED visits in previous year,c average no. per child (SD) | 2.14 (2.20) | 2.51 (2.38) | .17 |
| Inpatient hospitalizations in previous year,c average no. per child (SD) | 0.86 (0.95) | 0.93 (1.04) | .69 |
| Nighttime wheeze,d no. (%) | .47 | ||
| None | 42 (38) | 34 (41) | |
| 1–2 d/wk | 24 (22) | 19 (23) | |
| 2–4 d/wk | 11 (10) | 11 (13) | |
| Every day | 33 (30) | 19 (23) | |
| Nighttime cough,d no. (%) | .73 | ||
| None | 35 (32) | 23 (28) | |
| 1–2 d/wk | 12 (11) | 16 (19) | |
| 2–4 d/wk | 21 (19) | 17 (20) | |
| Every day | 42 (38) | 27 (33) | |
Note. ED = emergency department.
By the 2-sample t-test.
By the χ2 or Fisher exact test.
By the Wilcoxon rank-sum test.
By the Kruskal–Wallis test. For the immediate intervention group, n = 110; for the delayed intervention group, n = 83.
Caregiver Knowledge and Environmental Triggers
The goal of the intervention was to increase knowledge and change behaviors to promote reduced asthma triggers with a subsequent decrease in asthma morbidity. All participants showed a significant improvement in knowledge after being educated by lay health educators (P < .001). There was no significant difference (P = .45) between the immediate and delayed intervention groups. Asthma triggers were significantly reduced after the participants' completion of the environmental intervention. Dust antigen collection was completed at the initial visit for both groups, at the completion of the environmental intervention, and then again during the follow-up phase. Compared with the initial measurement, there was a significant reduction for Dermatophagoides pteronyssinus and Dermatophagoides farinae dust antigens after the intervention for both groups (P < .001).
There was also a significant difference seen in reduction of dust antigen between the immediate and delayed intervention groups. The delayed intervention group showed a greater reduction in dust antigen (P = .004); however, these data may be biased because there were more than twice as many bedrooms assessed for dust antigen after the intervention in the immediate intervention group (n = 81) than in the delayed intervention group (n = 35). This unequal sampling was the result of a problem with obtaining Aclotest kits during a period of the study.
Improved usage of both pillow and mattress covers to reduce dust mites exposure (Table 2) was significant after the intervention in both groups (P < .001). The odds of improvement of mattress and pillow cover use after the intervention were 380 and 496 times the odds without intervention. Reduction of roaches (P = .06) and rodents (P = .04) in the home were also significantly improved after the intervention for both the immediate and delayed intervention groups. Odds ratios (ORs) for improvement of these outcomes between before and after the intervention were 2.91 and 4.8, respectively (Table 2).
TABLE 2.
Effect of the Environmental Intervention on Trigger Improvement: Philadelphia, PA, 2002–2005
| Effect After Intervention Versus Before |
||
| Outcome | OR (95% CI) | P |
| Roach elimination or decrease | 2.91 (0.94, 9.06) | .06 |
| Rodent elimination or decrease | 4.8 (1.09, 21.23) | .04 |
| Smokers or smoking eliminated in home | 3.07 (0.4, 25.79) | .30 |
| Furry pets taken away from home | 1.36 (0.32, 5.81) | .68 |
| Bedroom carpet removed and replaced with tile | 1.29 (0.86, 1.93) | .21 |
| Mattress cover used | 380 (108, 1337) | < .001 |
| Pillow cover used | 496 (122, 2021) | < .001 |
Note. OR = odds ratio; CI = confidence interval. The generalized estimating equation method was used.
In analyzing period 1 alone, we modeled the effects of time and group on triggers in the immediate and delayed intervention groups. For smoking, roaches, rodents, and furry pets, there was no significant reduction over time (P = .56, P = .61, P = .10, and P = .60, respectively) and no significant difference between the 2 groups. Use of mattress and pillow covers increased over time (P < .001), with the immediate intervention group having a greater increase in use (OR = 488 and OR = 1310, respectively).
Asthma Symptoms and Medication Use
The primary outcomes were differences in the frequency of symptoms throughout the study and within-patient differences in emergency department and inpatient visits the year after the intervention compared with the year before the intervention.
Nighttime asthma symptoms and medication usage were analyzed for both the immediate and delayed intervention groups over time after intervention completion with controls for age group and baseline effects (Table 3). At baseline, there was a large range in the number of days of symptoms and medicine use observed. For the immediate intervention group, the mean number of days for nighttime cough was 9.6 ±9.39 days, for nighttime wheeze was 7.42 ±9.01 days, and for albuterol use was 11.79 ±10.2 days. The findings for the delayed intervention group were similar. For all 3 symptom variables analyzed (nighttime cough, nighttime wheeze, and albuterol usage), a significant baseline effect was observed; that is, although their symptoms decreased after the intervention, those participants who had the higher level of symptoms in the beginning still had a higher level at the end of the study (Table 3). However, after we controlled for this baseline effect, a significant decrease in nighttime wheeze was still observed in both the immediate and the delayed intervention groups over time after the educational and environmental intervention was completed (P < .001). Nighttime cough also decreased significantly over time after the intervention for the immediate intervention group (P < .001) and with borderline significance in the delayed intervention group (P = .08). Albuterol usage did not decrease significantly for either group after we controlled for the baseline effect and age group (2–4 years, 5–11 years, and ≥ 12 years) effect. We estimated that nighttime cough was reduced by 2 days and nighttime wheezing by 2.4 days for the immediate intervention group over 1 year. Nighttime cough was reduced by approximately 3 days for the delayed intervention group, and nighttime wheezing by about 5 days over 1 year. Albuterol use did not really change in the immediate intervention group; in the delayed intervention group, albuterol usage was reduced by about 2 days over 1 year. Age classification did not significantly impact symptoms, but there was a trend in nighttime symptoms decreasing as age increased in the immediate intervention group.
TABLE 3.
Nighttime Symptoms and Albuterol Usage After the Intervention: Baseline, Time, and Age Effects: Philadelphia, PA, 2002–2005
| Nighttime Cough |
Nighttime Wheeze |
Albuterol Use a |
||||
| Measure | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P |
| Immediate intervention group (n = 107) | ||||||
| Baseline | 0.31 (0.05) | < .001 | 0.31 (0.05) | < .001 | 0.33 (0.06) | < .001 |
| Time, mo | −0.17 (0.05) | < .001 | −0.2 (0.04) | < .001 | −0.01 (0.06) | .890 |
| Age group | ||||||
| 2–4 y | 2.6 (2.1) | .466 | 1.77 (1.9) | .634 | 1.7 (2.52) | .712 |
| 5–11 y | 2.22 (2.1) | 1.35 (1.89) | 0.89 (2.51) | |||
| ≥ 11 y | Reference | Reference | Reference | |||
| Delayed intervention group (n = 78) | ||||||
| Baseline | 0.48 (0.07) | < .001 | 0.63 (0.08) | < .001 | 0.52 (0.08) | < .001 |
| Time, mo | −0.23 (0.13) | .082 | −0.42 (0.11) | <.001 | −0.16 (0.15) | .298 |
| Age group | ||||||
| 2–4 y | −4.05 (2.95) | .391 | −3.27 (2.65) | .473 | −4.07 (3.23) | .275 |
| 5–11 y | −3.74 (2.88) | −2.83 (2.59) | −4.92 (3.1) | |||
| ≥ 11 y | Reference | Reference | Reference | |||
Note. A linear mixed model was used for analysis.
For the albuterol use measure in the immediate intervention group, n = 105.
In period one, nighttime cough, nighttime wheeze, and albuterol use all decreased over time in both groups (P < .001 for all variables). There was no significant difference between groups for nighttime cough and wheeze (P = .111 and P = .317, respectively). However, there was a trend in the immediate intervention group toward greater reduction in albuterol use over time (P = .09).
Utilization of Health Care Resources
Using data gathered from the hospital database, we totaled the number of asthma-related emergency department and inpatient visits both the year before the intervention and the year after the intervention separately. When we compared emergency department visits 6 months before the initial visit and 6 months after the initial visit, we saw that there was no statistical difference between the 2 groups (immediate intervention group, mean difference = 0.22 visits; delayed intervention group, mean difference = 0.33 visits; P = .98). When we compared inpatient visits 6 months before the intervention and 6 months after, we also found no difference between the 2 groups (immediate intervention group, mean difference = 0.14 visits; delayed intervention group, mean difference = 0.33 visits; P = .47), although both groups had fewer visits after intervention.
As shown in Table 4, a significant decrease (P < .001) in both emergency department visits and inpatient visits occurred in the study population the year after the intervention (active phase) compared with the year before the intervention after control for treatment group and age of child. For the immediate intervention group, the mean number of emergency department visits 1 year before enrollment was 2.26 ±2.27, and the mean number of inpatient visits was 0.85 ±0.99. At 1 year after enrollment, the mean number of emergency department visits was 1.72 ±2.28, and the mean number of inpatient visits was 0.48 ±0.86. This was similar for the delayed intervention group. As expected, no significant difference was observed between the immediate and delayed intervention groups. In general, younger children had significantly more emergency department visits and inpatient visits than did older children (P = .01 and 0.08, respectively, for emergency department and inpatient visits). In summary, after participation in the active phase, the mean number of emergency department visits per year per participant for the total study population decreased 30% from 2.3 to 1.6 (P < .001), and the mean total inpatient visits decreased by 53% from 0.89 to 0.43 (P < .001).
TABLE 4.
Changes in the Number of Emergency Department and Inpatient Visits: Philadelphia, PA 2002–2005
| Immediate Intervention Group (n = 118) |
Delayed Intervention Group (n = 85) |
Effect a |
|||||
| No. of Visits Year Before Intervention, Mean (SD) | No. of Visits Year After Intervention, Mean (SD) | No. of Visits Year Before Intervention, Mean (SD) | No. of Visits Year After Intervention, Mean (SD) | No. of Visits, Immediate Vs Delayed Intervention, Mean ±SD (P) | No. of Visits, Before Versus After Intervention, Mean ±SD (P) | No. of Visits, Age Group Effect, Mean ±SD (P) | |
| Emergency department visits | 2.26 (2.27) | 1.72 (2.28) | 2.35 (2.44) | 1.38 (1.69) | 0.02 ±0.13 (.89) | −0.38 ±0.09 (< .001) | |
| Age 2–4 y vs. age ≥ 12 y | 0.25 ±0.26 (.33) | ||||||
| Age 5–11 y vs. age ≥ 12 y | −0.17 ±0.26 (.50) | ||||||
| Inpatient visits | 0.85 (0.99) | 0.48 (0.86) | 0.95 (1.00) | 0.37 (1.00) | −0.04 ±0.16 (.81) | −0.72 ±0.15 (< .001) | |
| Age 2–4 y vs. age ≥ 12 y | 0.30 ±0.28 (.28) | ||||||
| Age 5–11 y vs. age ≥ 12 y | −0.06 ±0.29 (.85) | ||||||
Estimated difference, by the generalized estimating equation method with Poisson distribution.
DISCUSSION
In this study, we showed that an educational and environmental intervention delivered by lay health educators, whether immediate or delayed, was effective in reducing certain triggers, nighttime symptoms, and inpatient and emergency department use among urban children with asthma and a history of poor symptom control.
Our study corroborates the report of a randomized controlled trial from Morgan et al.8 In that study, the group that received education from a lay health educator as well as mattress and pillow covers and pest control, experienced a significant decrease in the number of days with asthma symptoms compared with the control group. Fewer emergency department visits were observed as well, but the difference was not significant. A study by Martin et al. 11 also showed that educational and environmental interventions delivered by a lay health educator were effective in reducing the number of environmental asthma triggers in both children and adults from a low-income Latino community. However, retention rates were considerably lower with 57% of children and 45% of adults completing the study, and no effect was observed on asthma severity or emergency department and hospital visits. Carter et al. 12 completed home visits on 147 children randomly assigned into 3 groups (intervention, control, and placebo) and followed for 1 year. No effect was seen in acute visits with the home environmental intervention, and they did not report on symptoms.
Relative to other studies, our study population, which consisted of a predominantly African American cohort, had a higher utilization of the emergency department and fewer working caregivers at enrollment. However, we did not have a true control group for the entire period as a result of the crossover design. We were able to compare the outcomes at 6 months with the delayed group serving as a control group. Interestingly, we did not observe differences between the 2 groups at 6 months. It appears that 6 months may be too soon to see a reduction in most of the triggers, except mattress and pillow covers. This is most likely because the mattress and pillow covers were given directly to the family, and the lay health educators assisted in their placement. By contrast, the other supplies required the caregiver to perform the implementation on his or her own.
Emergency department visits and inpatient visits were not reduced significantly when compared with 6 months before the initial visit, which may be attributed to the seasonal nature of asthma. It is difficult to explain why there were not many differences observed between the 2 groups at 6 months. One explanation is that the seasonal nature of asthma makes it difficult to evaluate changes in less than 1 year. Another explanation is that the monthly interaction between the lay health educators and the delayed intervention group may have promoted feelings of self-efficacy, increased attention to asthma symptoms, and therefore better asthma management. We did not measure smaller changes such as better cleaning habits, which could have occurred with the delayed intervention group and would have reduced exposures to dust and cockroach antigens. We did not measure smaller changes such as better cleaning habits, which could have occurred with the delayed intervention group and would have reduced exposures to dust and cockroach antigens. Finally, it is possible that there was a greater rate of change in the last 6 months, which would explain the differences seen at the end of the study.
It is important to note, however, that this intervention successfully reduced triggers, symptoms, and health care utilization for both groups. The longer the groups were in the study, the better the outcomes. Future studies are needed to explore qualitative benefits in interactions between lay health educators and caregivers.
Interestingly, there was a trend in the delayed intervention group toward better outcomes in most of the parameters at the end of the study. There may be a few explanations for these findings. First, this was our second intervention in the West Philadelphia community, and our lay health educators found that caregivers were already aware of the Community Asthma Prevention Program by word-of-mouth. Although the consent clearly stated the differences between the 2 groups, caregivers in the delayed intervention group complained on a regular basis of having to wait for 6 months before receiving the intervention. Subsequently, we had a much higher dropout rate in the delayed intervention group than in the immediate intervention group, although our retention rate was still higher than in many similar studies. Those families that did remain in the study were perhaps highly motivated, which may have led to them making changes in their environment before the intervention.
This study was not designed to calculate cost-effectiveness. Relative to other studies that reported similar outcomes, however, the total costs for the environmental and educational intervention were relatively low at $121 per child enrolled. This did not include the cost of the staff time, which would raise the cost to approximately $450 to $500 per family. In a nonresearch setting, these costs would be even less because the lay health educators would be able to carry double the caseload. Other studies have reported costs in similar interventions ranging from $189 to $1469.13-15 Thus, given that our outcomes were similar to these studies, this intervention is relatively low-cost and has cost-benefit implications for payers to utilize trained lay health educators to provide educational, environmental, and social support services to urban, disadvantaged families to reduce the asthma burden on these same families. Asthma is at epidemic proportions in urban, disadvantaged communities, and, as evidenced in this study, reduction of emergency department visits and inpatient visits by 40% to 60% was largely the result of the attention and social support provided by the lay health educators. Thus, this is a low-cost model that could potentially have a large impact on asthma health care costs.
Limitations
Our study did have limitations. The randomized crossover design, although enabling within-group comparisons, limited our ability to draw conclusions between the 2 groups because a carryover effect was measured in the immediate intervention group during the nonintervention period. However, in accordance with the environmental justice approach, the community's input in the research design was as important as the researchers' input. A randomized parallel group design was not acceptable to the community because some participants would not receive the intervention. Also, the community thought involvement of more than 1 year in the study would create a burden on participants. To bridge the gap between the researchers and the community, a randomized crossover design with a 6-month inactive period preceding the intervention was used to create conditions as close as possible to a randomized controlled, parallel design. Another limitation is that whereas the Aclotest kit seemed ideal because it allowed immediate analysis and reduced the costs of dust analysis as well as the burden on the lay health educators, it became unavailable during the intervention for the delayed intervention group. Hence, fewer homes were assessed for follow-up, which limited the robustness of our conclusions regarding dust. Additionally, we did not measure cost-effectiveness, but we were able to do a cost analysis based on intention-to-treat. Furthermore, we targeted the environmental exposures rather than performing skin testing on individuals. Others have shown that focusing interventions based on skin testing is effective in reducing asthma symptoms.8,12 By focusing on exposures instead of skin testing, however, we increased the pool of eligible children by removing barriers to enrollment and also reduced the cost. In terms of impact, this generalized approach is easier to disseminate.
There were many lessons learned from our study. As trained community residents, the lay health educators were instrumental in recruiting and implementing this project. Parents readily identified with the lay health educators and they were perceived as empathetic and flexible. The lay health educators were also able to provide valuable feedback to the research team in terms of the feasibility, burden, and challenges of the intervention. The lay health educators made the final decision about whether families were lost to follow-up and were very committed to keeping families involved. Although the crossover design allowed the intervention to begin in the delayed intervention group at 6 months rather than 1 year, the lay health educators found keeping the delayed intervention group families involved for more than 1 year after enrollment to be very difficult, which reduced our retention rate.
Environmental and social issues are significantly different in urban populations than in the general population, and continued research is needed to develop culturally appropriate strategies for environmental mitigation and asthma management among urban asthmatics, particularly children.16 As an extension of this work, the Philadelphia Community Asthma Prevention Program has undertaken a multifaceted intervention using the community-based participatory approach to combine the home visit environmental mitigation with practice-specific training for primary physicians focusing on implementation of the National Heart, Lung, and Blood Institute guidelines on asthma management, education for school professionals to improve asthma management at school, and caregiver and patient education delivered through community agencies and schools, respectively.
Conclusions
Educational and environmental interventions conducted during home visits significantly improved caregiver knowledge and created an environment with significantly fewer common asthma triggers for the asthmatic child. Decreases in some asthma symptoms and emergency department visits and hospitalizations occurred during the period when the participants' homes were visited by community lay health educators. Delivery of education and environmental interventions by lay health educators was well accepted among families of urban, disadvantaged children with asthma and may be a cost-effective method for reducing the burden of asthma within this population.
Acknowledgments
This study was funded by the National Institute for Environmental Health Sciences, (grant 1 R25 ES11115-01).
We acknowledge the lay health educators Beverly DeVignes, Charmane Braxton, and Sherry Biggs for their tireless efforts in this study. We acknowledge Galen Laprocido, who helped to design and implement this study as project manager. We acknowledge Heather Haley for medical writing assistance. Most important, we'd like to acknowledge all of the caregivers and children who participated in this study.
Human Participant Protection
This study was reviewed and approved by the institutional review board of the Joseph Stokes Research Institute, the Children's Hospital of Philadelphia.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1–13 [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, et al. National Surveillance for Asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1–14, 18-54 [PubMed] [Google Scholar]
- 3.Smedley BD, Stith AY, Nelson AR, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington DC: National Academies Press; 2003 [PubMed] [Google Scholar]
- 4.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart Lung Blood Institute; 2007 [Google Scholar]
- 5.Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low-income urban children: preliminary findings from the Seattle-King County health homes project. J Urban Health. 2000;77(1):50–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant-Stephens T, Li Y. Outcomes of a home-based environmental remediation for urban children with asthma. J Natl Med Assoc. 2008;100(3):306–316 [DOI] [PubMed] [Google Scholar]
- 7.Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524 [DOI] [PubMed] [Google Scholar]
- 8.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environment intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080 [DOI] [PubMed] [Google Scholar]
- 9.Evans R., III A randomized clinical trial to reduce asthma morbidity among inner-city children; results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338 [DOI] [PubMed] [Google Scholar]
- 10.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9(2):128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MA, Hernandez O, Naureckas E, Lantos J. Reducing home triggers for asthma: the Latino community health worker approach. J Asthma. 2006;43:369–374 [DOI] [PubMed] [Google Scholar]
- 12.Carter MC, Perzanowsi MS, Raymond A, Platts-Mills TAE. Home intervention in the treatment of asthma among inner-city children. J Allergy Clin Immunol. 2001;108:732–737 [DOI] [PubMed] [Google Scholar]
- 13.Krieger JW, Takatro TK, Song L, Weaver M. The Seattle-King County Health Homes Project: a randomized controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan SD, Weiss KB, Lynn H, et al. National Cooperative Inner-City Asthma Study (NCICAS) Investigators. The cost-effectiveness of an innercity asthma intervention for children. J Allergy Clin Immunol. 2002;110:576–581 [DOI] [PubMed] [Google Scholar]
- 15.Kattan M, Stearns SC, Crain EF, et al. Cost-effectiveness of a home-based environmental intervention of inner-city children with asthma. J Allergy Clin Immunol. 2005;116:1058–1063 [DOI] [PubMed] [Google Scholar]
- 16.Swartz LJ, Callahan KA, Butz AM, et al. Methods and issues in conducting a community-based environmental randomized trial. Environ Res. 2004;95:156–165 [DOI] [PubMed] [Google Scholar]