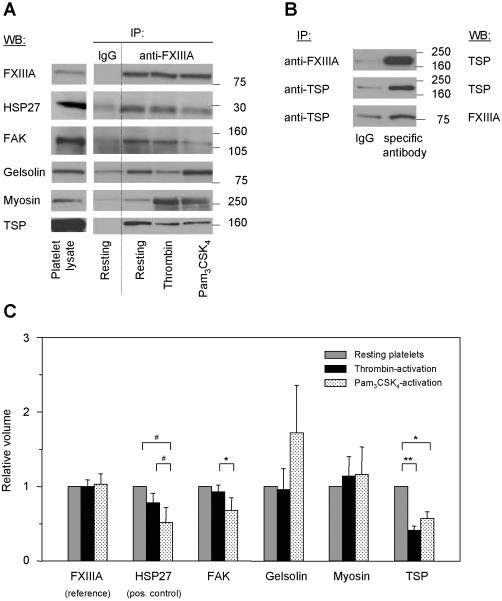
Figure 5. Validation of FXIIIa-binding proteins by immunoblotting and coimmunoprecipitation, and quantitative analysis of the FXIIIa-protein interactions.
(A) Proteins identified by 1D- and 2D-GE, MALDI-TOF MS and PMF analysis were tested by immunoblotting for the specificity and intensity of their FXIIIa-interaction. FXIIIa was immunoprecipitated at an 1:250 antibody:protein ratio from lysates of resting and activated platelets (all lanes “anti-FXIIIa”). As control, a non-specific antibody was used (lanes “IgG”, “Resting”). All immunoprecipitates were separated by 1D-GE, transferred and blotted for the identified proteins listed in Table 1. Here, representative immunoblots for the four specific FXIIIa-binding proteins only are shown. HSP27, as a known FXIIIa-interacting protein, was included as positive control. Lane “Platelet lysate” shows the position of the protein in the total lysate of resting platelets. (B) The interaction between FXIIIa and thrombospondin was tested by co-immunoprecipitation and immunoblotting in resting platelets. The FXIIIa-immunoprecipitate was separated by 1D-GE and blotted for thrombospondin (top image). Thrombospondin was immunoprecipitated with a specific antibody, separated by 1D-GE and blotted first for thrombospondin (middle image) and then for FXIIIa (bottom image). As control, corresponding non-specific antibodies (lanes “IgG”) were used. (C) To determine agonist-induced changes in FXIIIa-protein interactions, quantitative analysis of the immunoblot images in Figure 5A was performed by densitometry for all lanes “anti-FXIIIa”. Band intensities were quantified for each protein, then normalized to the band intensity of FXIIIa and to the protein's band intensity in resting platelets (see also Table 2). ** indicates p < 0.01; *, p < 0.05; #, p = 0.07; IP, immunoprecipitation; IB, immunoblot; TSP, thrombospondin; and FAK, focal adhesion kinase.