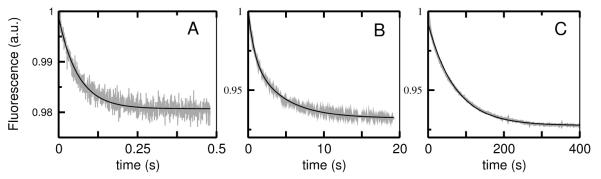
FIGURE 3.
Kinetics of dissociation of mastoparan X (masX) from vesicles of POPC (A), POPS/POPC 20:80 (B), and POPS/POPC 50:50 (C), at room temperature (about 22°C). The signal shows the decrease in FRET from the Trp of the peptide to 7MC-POPE as masX dissociates from the membrane. The experimental data is shown in gray (the examples in A and B are averages of 4–7 traces of 50 μM total lipid; in C, 1 trace is shown). The signal-to-noise ratio is larger the better the binding, as the content of PS in the vesicles increases (A<B<C). The solid black line is a single-exponential fit in A and C, and a double-exponential fit in B. The mean, apparent rate constant provides an estimate of koff. For dissociation from POPS/POPC 50:50 (C), because of the long measurement time, other, much slower processes are also detected, probably including photobleaching and vesicle fusion, which is more likely if the anionic lipid content is high. These slow processes can be well described by an exponential decay with a time constant of about 1000 s, which was subtracted from the curve shown.