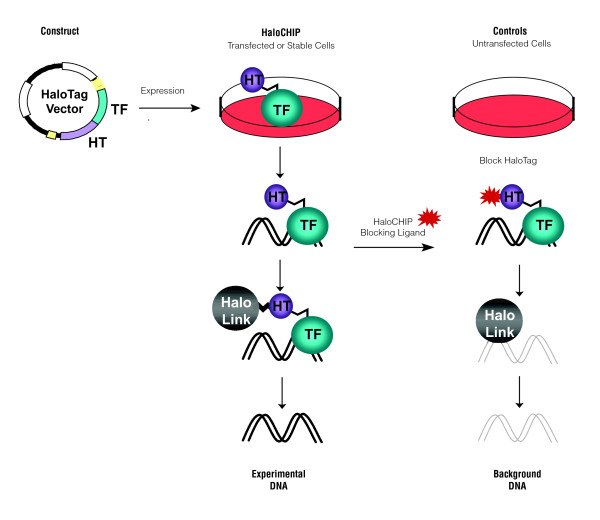
Figure 1.
Schematic of the HaloCHIP process. The HaloCHIP process is initiated by cloning a desired DNA binding protein-of-interest, i.e. a transcription factor (TF) into a HaloTag (HT) fusion mammalian expression vector. For the experimental HaloCHIP sample, the HaloTag fusion protein is expressed in the desired cell line and then crosslinked to DNA in vivo with formaldehyde. Treated cells are lysed, sonicated to shear the chromatin, and incubated with HaloLink resin, which directly and covalently captures all crosslinked HaloTag-fusion complexes. The resin is stringently washed to remove non-specific proteins and DNA, and captured regions of DNA are released by reversal of the crosslinks. The resultant DNA can be further purified for downstream analysis. Two possible controls, which provide an estimate of background DNA capture, are recommended for the HaloCHIP process. The first, referred to in the text as the untransfected control, involves the use of untransfected cells which are processed in parallel with the HaloCHIP experimental sample. The second, referred to as the blocking ligand control, is generated by splitting the cell lysate equally into separate tubes prior to incubation with the HaloLink resin and incubating the control sample only with a fluorescent HaloCHIP blocking ligand, preventing interaction of the HaloTag complexes with the HaloLink resin during the subsequent incubation step.