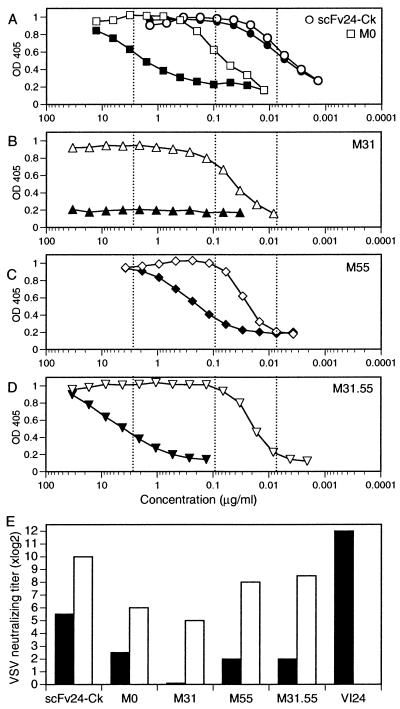
Figure 3.
VSV-specific binding and neutralization of scFv-Cκ antibody fragments. Supernatant containing scFv-Cκ protein was serially 2-fold diluted and transferred to VSV-coated plates. Bound scFv-Cκ was detected by horseradish peroxidase-labeled anti-Cκ antibody (closed symbols). To determine avidity effects, supernatant containing scFv-Cκ was crosslinked by horseradish peroxidase-labeled anti-Cκ antibody before serial 2-fold dilution and then applied to VSV-coated plates (open symbols). (A) VSV-specific binding of hypermutated scFv24-Cκ (circles) and of germ-line scFv-Cκ M0 (squares). (A–D) Half-maximal binding of scFv24-Cκ and M0 is indicated by dotted lines. (B) The VH Ser31-to-Asn substitution did not improve scFv-Cκ binding. (C) Despite the fact that the VH Ser55-to-Arg substitution was not expressed by the parental antibody VI24, it improved the binding of M55. (D) Coexpression of the VH Ser31-to-Asn and the VH Ser55-to-Arg substitutions improved binding of M31.55. (E) VSV-IND neutralization by various scFv-Cκ antibody fragments was tested with untreated reagents (black bars) and after crosslinking (open bars). For crosslinking, 2.5 μg of purified scFv-Cκ protein was incubated with 1.25 μg of anti-Cκ antibody in a total volume of 100 μl for 30 min at room temperature. The crosslinked and untreated scFv-Cκ protein and the purified antibody VI24 were serially 2-fold diluted and analyzed in a standard neutralization assay. The protein dilution reducing the number of plaques by 50% is indicated as the titer.