Abstract
Electrical interaural time delay (ITD) discrimination was measured using 300-ms bursts applied to binaural pitch matched electrodes at basal, mid, and apical locations in each ear. Six bilateral implant users, who had previously shown good ITD sensitivity at a pulse rate of 100 pulses per second (pps), were assessed. Thresholds were measured as a function of pulse rate between 100 and 1,000 Hz, as well as modulation rate over that same range for high-rate pulse trains at 6,000 pps. Results were similar for all three places of stimulation and showed decreasing ITD sensitivity as either pulse rate or modulation rate increased, although the extent of that effect varied across subjects. The results support a model comprising a common ITD mechanism for high- and low-frequency places of stimulation, which, for electrical stimulation, is rate-limited in the same way across electrodes because peripheral temporal responses are largely place invariant. Overall, ITD sensitivity was somewhat better with unmodulated pulse trains than with high-rate pulse trains modulated at matched rates, although comparisons at individual rates showed that difference to be significant only at 300 Hz. Electrodes presenting with the lowest thresholds at 600 Hz were further assessed using bursts with a ramped onset of 10 ms. The slower rise time resulted in decreased performance in four of the listeners, but not in the two best performers, indicating that those two could use ongoing cues at 600 Hz. Performance at each place was also measured using single-pulse stimuli. Comparison of those data with the unmodulated 300-ms burst thresholds showed that on average, the addition of ongoing cues beyond the onset enhanced overall ITD sensitivity at 100 and 300 Hz, but not at 600 Hz. At 1,000 Hz, the added ongoing cues actually decreased performance. That result is attributed to the introduction of ambiguous cues within the physiologically relevant range and increased dichotic firing.
Keywords: binaural hearing, auditory prosthesis, auditory models
Introduction
Stimulation of the auditory nerve using bilateral cochlear implants (BiCIs) offers well-documented improvements over unilateral device use in sound localization and speech understanding in spatially distinct noise (e.g., van Hoesel and Tyler 2003; Nopp et al. 2004; Litovsky et al. 2006; Neuman et al. 2007; Grantham et al. 2007). Whereas listeners with normal hearing derive substantial benefit from low-frequency interaural time delays (ITDs; e.g., Levitt and Rabiner 1962; Wightman and Kistler 1992) in addition to level differences at the two ears, BiCI users are restricted predominantly to the latter (van Hoesel and Tyler 2003; van Hoesel 2004; Grantham et al. 2007). While that is as expected with present clinical sound processors that discard fine-timing cues, the same was reported for a research processor in which those cues were explicitly coded (van Hoesel et al. 2008). Psychophysical data with small numbers of BiCI users (van Hoesel and Tyler 2003; van Hoesel 2004; Majdak et al. 2006; Laback et al. 2007; van Hoesel 2007, 2008) have shown that at pulse rates or modulation rates of 100 Hz, listeners with good sensitivity can hear ITDs of around 100 μs, as can normal-hearing listeners attending 100-Hz pure tones (Klumpp and Eady 1956). As rate increases, ITD sensitivity becomes worse in BiCI users. In normal-hearing listeners, that is also the case for high-frequency envelope ITDs, but for low-frequency pure tones, sensitivity instead improves up to 1 kHz (Klumpp and Eady 1956). Colburn and Equissaud (1976) suggested that the difference between high- and low-frequency regions in normal hearing is due to the differences in peripheral processing rather than separate ITD processing mechanisms. Using transposed tones (van der Par and Kohlrausch 1997), high-frequency envelopes can be created that match the half-wave-rectified magnitude response to low-frequency pure tones. ITD sensitivity with those two signals has indeed been shown to be very similar, but only up to rates of about 150 Hz (Bernstein and Trahiotis 2002; Oxenham et al. 2004), beyond which, high-frequency envelope cues become less salient. The divergence at higher rates may be due to remaining peripheral differences, such as the phase relations of responding neurons around the characteristic place being activated, which correspond to monotonic traveling wave delays matched to the pure tone frequency, but not the envelope fluctuation rate for the transposed tone. Alternatively, it may yet reflect fundamentally different processing of ITDs for the two regions. The difference in peripheral response for high- and low-frequency regions due to mechanical frequency coding is eliminated with electrical stimulation, and the present work therefore affords further opportunity to assess whether ITDs in different frequency regions are processed similarly. Electrical ITD thresholds were determined both for unmodulated and modulated bilateral pulse trains applied to apical, mid, and basal regions along the electrode arrays.
The limited range of rates over which ongoing envelope timing cues are available in normal hearing is also reflected in the increased dominance of the onset ITD at higher rates (e.g., Hafter and Dye 1983; Saberi 1996; Stecker and Hafter 2002). Onset dominance at higher rates for electrical stimulation has similarly been indicated in recent studies. The data from Laback et al. (2007) with brief four-pulse stimuli showed increasing sensitivity to the onset ITD in the presence of diotic post-onset pulses as pulse rate increased. The study by van Hoesel (2007) demonstrated that (a) adding more pulses to variable duration signals produces much less reduction in threshold at 400 pulses per second (pps) than at 100 pps, (b) binaural beats produced by steadily increasing ITDs in signals with diotic onsets are heard over a much more limited range of rates than that for which static ITDs are detectable, and (c) ITDs applied to the first pulse in a two-pulse stimulus are more readily detected than when applied to the second pulse when the two pulses are separated by only a few milliseconds. Direct measurement of the relative weights of electrical ITD cues applied to each pulse in two- and eight-pulse sequences was described in van Hoesel (2008) and showed strongly reduced post-onset weights for ITDs at 300 and 600 pps, but much less so for interaural level differences. The present work assesses the role of onset and ongoing ITD cues in signals with longer duration and over a wider range of rates. ITD thresholds were determined for single-pulse stimuli and compared with those for 300-ms bursts with zero rise time for pulse rates and modulation rates up to 1,000 Hz. In addition, thresholds for 300-ms bursts at 600 Hz were measured for signals with a slower linear ramp of 10 ms.
Methods
Subjects and electrode selections
Six BiCI users were selected for good ITD sensitivity at a low pulse rate of 100 pps from a larger pool of listeners assessed previously (Litovsky et al., submitted). All six were well acquainted with the listening tasks administered. Five of the six listeners experienced hearing loss as adults and one during mid-childhood. All but one also had more than two and a half years of experience listening to bilateral cochlear implants, albeit using clinical processors that code only envelope timing cues. Some relevant details regarding hearing loss history and implant experience are provided in Table 1. Listeners were tested over 2- or 3-day periods at the University of Wisconsin. Occasionally, additional visits were needed to complete the set of measurements or retest some subjects.
TABLE 1.
Selected details for the participating listeners: electrodes used (matched pitch), hearing loss (HL) history, cochlear implant (CI) and hearing aid (HA) experience
| Subject | Electrodes tested (apical,mid,basal) | Age at test (years) | Age at HL onset (yrs) | Type of HL | Etiology | Duration CI use (years) | HA use | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | ||||
| IAD | 20,12,4 | 20,12,6 | 49 | 18 | 46 | Sudden | Unknown | 2.5 | 2.5 | No | No |
| IAK | 21,12,6 | 20,14,4 | 59 | 27 | 27 | Sudden | Ototoxicity | 15 | 3 | Yes | Yes |
| IAN | 20,14,6 | 20,14,4 | 56 | 38 | 38 | Progressive | Hereditary | 1.5 | 8 | Yes | Yes |
| IAP | 20,12,4 | 20,12,4 | 65 | 24 | 24 | Progressive | Meniere’s | 6 | 6 | Yes | Yes |
| IAR | 20,12,6 | 20,12,6 | 51 | Child | Child | Progressive | Unknown | 4 | 5 | Yes | Yes |
| IAW | 20,14,6 | 22,14,4 | 83 | 28 | 28 | Progressive | Hereditary | 0.85 | 7 | Yes | Yes |
All six participants were implanted with Nuclues 24 devices incorporating electrode arrays with 22 electrodes separated by 0.75 mm. Three bilateral electrode pairs were selected for each subject at apical, mid, and basal locations along the array. While actual insertion angles will have varied somewhat among subjects, a typical full insertion positions the apical bands at or beyond 300°, corresponding to frequencies of 1,000 Hz or lower on the cochlear spiral ganglion frequency map (e.g., Stakhovskaya et al 2007). Assuming conservatively an angle of 300° for the apical bands, mid array electrodes will excite the 2- to 3-kHz region and the basal ones the region around 5–6 kHz. Place of stimulation was matched between ears at 100 pps in the previous study mentioned above (Litovsky et al., submitted) using a pitch magnitude estimation task followed by pairwise comparison, as described in van Hoesel (2007). The resulting electrode bands, numbered in a basal to apical direction, are described in Table 1. Only modest offsets were found between ears, with selected pairs differing by no more than two electrodes (1.5 mm).
Stimuli and task
Electrical stimuli comprised biphasic monopolar current pulses of 25 μs per phase with an interphase gap of 8 μs. Stimuli were generated via direct computer control of the implants in both ears using a custom research platform based on a SPEAR3 research processor (CRC HEAR, Australia) and dedicated psychophysics software, thus ensuring interaural timing accuracy of 2 μs or better. On each trial within an experimental block, listeners were presented with a zero-ITD “reminder” stimulus, followed by a 500-ms silent interval, and then the stimulus containing either a left- or right-leading ITD cue and were required to respond whether the stimulus was to the left or right of the reminder. Each test block included four ITD magnitudes spaced in equal intervals on a logarithmic scale between 100 and 800 μs. Each cue magnitude was repeated 40 times within a test block (with half the cues to the left and half to the right). For each ITD magnitude, responses to left- and right-leading ITD stimuli were used to calculate hit and false alarm rates. Scores of 0% or 100% were converted to 100/2N and 100 − 100/2N %, respectively (where N is the number of repeat presentations). Those scores were subsequently used to calculate bias-corrected percent correct scores [P(C)max] (MacMillan and Creelman 2005), and a normal cumulative Gaussian curve was fitted to the four ITD magnitude data points using weighted linear regression. Thresholds were determined at a performance level of P(C)max = 69%, corresponding to d prime (d′) = 1 in a yes–no task. The reported thresholds reflect twice the magnitude of the ITD applied to the left or right ear stimulus to allow for the possibility that listeners are able to take advantage of previous stimulus presentations containing ITDs leading in the opposite ear. Standard errors were estimated using a bootstrap procedure based on a 1,000-trial Monte Carlo simulation (Foster and Bischof 1991). If the range of cues contained in a test block did not include the estimated threshold, additional tests were completed with revised cue magnitudes as needed. Subjects were well trained on the task through participation in a previous study. No feedback was provided during data collection.
ITD sensitivity was assessed for both modulated and unmodulated pulse trains at all three places. Stimulus conditions were randomized in test order across subjects.
For the unmodulated stimuli, pulse rates of 100, 300, 600, and 1,000 pps were tested. Stimulation levels for unmodulated pulse trains were held fixed at that corresponding to approximately 90% of the dynamic range at 1,000 pps. If stimuli at different rates had instead been balanced for loudness, the anticipated poorer performance at higher rates might be due to the use of lower stimulation levels rather than rate per se because ITD sensitivity can be reduced at lower levels (van Hoesel 2007). For the modulated stimuli, pulse rate was held constant at 6,000 pps, and modulation rates were 100, 300, 600, and 1,000 Hz. Modulation shape was sinusoidal in terms of cochlear clinical units (CU, which are approximately logarithmic in terms of current), and the peak modulation depth was held fixed at 40 CU, corresponding to about 6.8 dB. For comparison, the dynamic range for unmodulated pulse trains at 6,000 pps ranged from 57 to 127 CU across these listeners, so that the modulation depth was between about 30% and 60% of their dynamic range. Note that in perceptual terms, the modulation depth was probably even greater because equal changes in CU can have a considerably larger effect on loudness at high levels than at lower ones. Overall stimulation levels for modulated signals were set at about 90% of the dynamic range for a signal with 100-Hz modulation. Starting from those levels, balance of levels across ears was adjusted at each rate or modulation rate to ensure stable, fused, and centered sound images. On rare occasions where some uncertainty existed in this regard, levels were further adjusted to ensure 800-μs ITDs applied to either ear resulted in equidistant lateralization shifts toward the non-delayed ear.
For the larger set of test conditions, pulse trains were gated on and off using a rectangular window, referred to as the “fast rise time” condition. To assess the good ITD sensitivity at 600 Hz shown by several subjects, additional measurements were made at that rate for the electrode pair that presented with the best ITD sensitivity in each listener. A 10-ms linear ramp (slow rise time) was applied to both onset and offset portions of the envelope, reducing initial and final stimulation levels to threshold. Measurements with rectangular gating were also repeated so that the order of testing with fast and slow rise times could be randomized across subjects. Duration of the pulse trains inclusive of either rise time was always 300 ms. Sensitivity to the onset ITD in isolation was assessed by measuring thresholds for single-pulse stimuli for each of the three matched-place electrode pairs and at the same levels as used in the 300-ms bursts. In all experimental conditions, ITDs were applied to the entire stimulus waveforms.
Results
Effect of pulse rate and modulation rate at three places
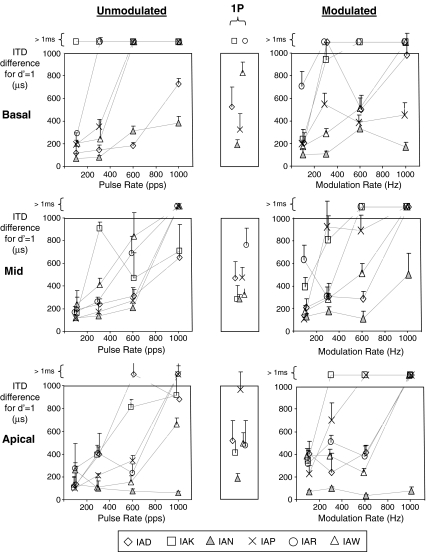
Figure 1 shows ITD thresholds for 300-ms stimulus bursts as a function of pulse rate (leftmost column) or modulation rate (rightmost column) for unmodulated pulse trains and modulated 6,000-pps pulse trains, respectively. The top, middle, and bottom rows in each column show data for basal, mid, and apical electrode pairs, respectively. Individual subject data are delineated using different symbols as described in the figure legend. Unmeasurable thresholds and those in excess of 1 ms, which reflect an inability to discriminate left from right ear delays when the magnitude of the delay exceeds 500 μs, are plotted above the graphs.
FIG. 1.
ITD thresholds for 300-ms stimulus bursts as a function of pulse rate (leftmost column) or modulation rate (rightmost column). The top, middle, and apical rows in each column show results for basal, mid, and apical electrode pairs, respectively. Individual subject data are shown by the different symbols. Unmeasurable thresholds or those in excess of 1 ms that reflect an inability to discriminate left from right ear delays when cues in each ear were 500 μs or more are shown above the graphs. Single-pulse ITD thresholds are plotted in the narrow central column on the same scale as for the 300-ms bursts.
Three separate analyses were applied to the data. First, an analysis of variance (ANOVA) was applied to the data set describing performance with both unmodulated and modulated 300-ms bursts. Data from the basal electrode pair for subject IAK were excluded as they were uninformative due to thresholds exceeding 1 ms at all rates. Fixed factors in the model included: (1) signal type, to delineate unmodulated and modulated stimuli; (2) rate, to describe pulse rates or modulation rates at 100, 300, 600, and 1,000 Hz; and (3) place, to denote apical, mid, or basal electrode pairs. Subjects were included as a random blocking factor. For the purpose of analysis, thresholds recorded as exceeding 1 ms were arbitrarily assigned a value of 1.1 ms. That value was likely a conservative estimate for most of those cases. While that approach will have been of little consequence for stimulus conditions generally leading to thresholds below 1 ms, it will have reduced differences among conditions resulting in poor performance. For that reason, a second ANOVA was applied to a reduced data set excluding the stimulus conditions leading to high thresholds, and a third analysis was completed using non-parametric tests.
Results from the first ANOVA showed a highly significant effect of rate [F(1,111) = 42.2, p < 0.001], a moderately significant effect of signal type [F(1,111) = 2.6, p = 0.017], no significant effect of place [F(2,111) = 2.6, p = 0.08], and no significant interactions between any of the factors. “Conservative mean thresholds” (CMTs) were calculated assuming a value of 1.1 ms for thresholds in excess of 1 ms. When CMTs were averaged across place and stimulus type, at rates of 100, 300, 600, and 1,000 pps, those means were 236, 449, 614, and 902 μs. The 5% least significant difference (LSD) was 85 μs, indicating that CMTs for all rate comparisons differed significantly despite the compressive effect of the 1.1-ms threshold limit. CMT thresholds for the two stimulus types, averaged across rate and place, were 498 μs for unmodulated stimuli compared to 603 μs for unmodulated ones, with a 5% LSD = 86 μs. Comparison between the two stimulus types at each rate showed that only the difference at 300 Hz was significant (unmodulated signal threshold 348 μs, modulated signal threshold 550 μs, 5% LSD = 172 μs). While overall the place effect was not significant in the ANOVA, the difference in CMTs for basal and apical electrodes was large enough to be significant. Mean thresholds for basal, mid, and apical electrodes were 613, 544, and 494 μs, with a 5% LSD of 105 μs.
The second ANOVA was applied to a reduced data set excluding most of the stimulus conditions that led to thresholds in excess of 1 ms. That criterion was satisfied by excluding the data for the two highest rates tested, as well as the basal electrode pairs for which several subjects showed very high thresholds even at the lower rate of 300 Hz. Results again supported all three conclusions from the first ANOVA: the effect of rate was shown to be highly significant [F(1,35) = 9.69, p = 0.004], the effect of stimulus type was moderately significant [F(1,35) = 6.85, p = 0.013], and that of place was not significant [F(1,35) = 0.01, p = 0.9]. As in the first ANOVA, interactions were all non-significant (at p = 0.05). It should be noted that because basal electrodes were excluded in this ANOVA, the effect of place in this case only assesses the difference between mid and apical electrodes.
Finally, non-parametric Kruskall–Wallis tests were applied to examine single-factor effects. To avoid excessive rank ties due to the hard limit of 1.1 ms, data entries were again discarded for rate–electrode combinations that resulted in thresholds exceeding 1 ms for both modulated and unmodulated signals. The results of that analysis were again in good agreement with the first ANOVA, demonstrating a strong effect of rate (H = 26.64, df = 3, p < 0.001), a more moderately significant effect of signal type (modulated versus unmodulated; H = 4.70, df = 1, p = 0.03), and non-significant effect of place (H = 0.67, df = 2, p = 0.714).
Overall, these analyses show (1) no significant effect of place, although there was an indication that performance for basal stimulation was slightly worse than the apical one; (2) a strong effect of rate with both modulated and unmodulated stimuli, showing better performance at lower rates for all three places; and (3) somewhat poorer performance with modulated than unmodulated signals at matched modulation and pulse rates, respectively.
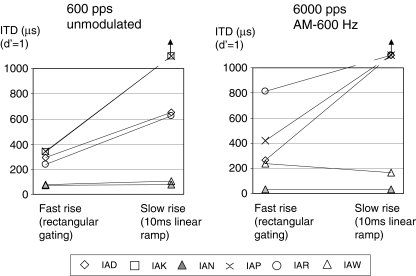
Onset rise time effects at 600 Hz
Figure 2 describes measured ITD thresholds for fast (rectangular gating) and slow (10-ms linear ramp) rise times at pulse rates (left panel) or modulation rates (right panel) of 600 Hz. Selected electrode pairs were those showing best performance at 600 Hz in Figure 1. Subject IAK could not be tested with modulated stimuli as none of the electrode pairs elicited ITD sensitivity below 1 ms at 600 Hz. A non-parametric Wilcoxon matched-pairs signed-ranks test showed a significant effect of the ramp rise time (S = 4, N = 11, p = 0.01) when results for both modulated and unmodulated signals were included in the data set. However, it is clear from Figure 2 that the two listeners with the best performance at 600 Hz, IAN and IAW, showed similar thresholds with either fast or the slow onset rise times. That implies that for those two subjects, ongoing cues in the 300-ms burst provided sufficient information for overall performance to be little affected by the absence of a strong onset cue.
FIG. 2.
ITD thresholds for fast (rectangular gating) and slow (10-ms linear ramp) rise times. The left panel shows results for unmodulated pulse trains at 600 pps and the right panel those for 6-kHz pulse trains modulated at 600 Hz. The electrode pairs used were those showing best performance at 600 pps (see Fig. 1). Subject IAK could not be tested with modulated stimuli as none of the electrode pairs elicited ITD sensitivity.
Single-pulse threshold comparison
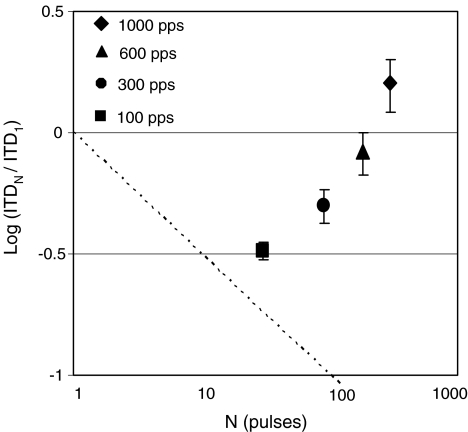
Single-pulse ITD thresholds are plotted in the narrow central column in Figure 1 on the same scale as for the 300-ms bursts, which at rates of 100, 300, 600, and 1,000 pps comprise 30, 90, 180, and 300 pulses, respectively. For subject IAN only, an error in the “single-pulse” procedure resulted in inadvertent presentation of two pulses separated by 300-ms rather than just one in each interval. If that listener benefited from ideal-observer-like performance gains (Green and Swets 1966) due to the addition of the second pulse, her true single-pulse thresholds may be up to 1.4 times higher than shown. Single-pulse ITD thresholds were compared with those for 300-ms bursts with fast rise times (Fig. 1, left column) at each pulse rate using a Wilcoxon matched-pairs signed-ranks test. Data from all three places along the electrode arrays were included. Results showed significantly lower thresholds for 300-ms bursts compared to the single pulses at 100 pps (S = 0, N = 17, p < 0.001) and 300 pps (S = 17, N = 16, p = 0.008), but not at 600 pps (S = 64, N = 16, p = 0.8). At 1,000 pps, thresholds were significantly higher for the bursts than for a single pulse (S = 6, N = 16, p = 0.001), indicating that the addition of post-onset information was disruptive to overall performance at that rate. Figure 3 shows the subject- and place-averaged improvements in ITD threshold when comparing 300-ms bursts and single-pulse stimuli. Results are plotted as the logarithm of that ratio as a function of the number of pulses in the 300-ms burst (which increases linearly with pulse rate). If subjects behaved as ideal observers, improvements with increased duration would fall along the dashed negative diagonal (e.g., Hafter and Dye 1983). The data show that was approximately the case only at 100 pps and increasingly less so as rates increased. The positive value at 1,000 pps indicates the poorer ITD sensitivity with the 300-ms burst than with a single pulse at that rate.
FIG. 3.
Filled symbols show changes in ITD thresholds for 300-ms unmodulated bursts relative to those for single-pulse stimuli averaged over subjects and electrodes. Error bars show standard errors of the means. Results are plotted as the logarithm of the ratio of the thresholds for 300-ms bursts to those for single pulses as a function of the number of pulses in the 300-ms burst (which increases linearly with pulse rate). For comparison, ideal observer performance, which would improve as the square root of the number of pulses, is shown by the dashed negative diagonal.
The extent to which single-pulse ITD thresholds are predictive of those for the 300-ms unmodulated bursts with fast rise times was also tested using an analysis of covariance (ANCOVA) model. Single-pulse thresholds were used as a covariate describing 300-ms burst thresholds as a function of place and rate (and their interaction), with subjects again treated as a random factor. Results showed that for the full data set, the single-pulse thresholds were a significant predictor [F(1,54) = 5.97, p = 0.018] of the burst thresholds. However, when subject–electrode combinations that showed single-pulse thresholds in excess of 1 ms were omitted, the prediction was no longer significant [F(1,46) = 0.14, p = 0.7]. Similar findings were supported by Spearman rank correlation analysis that showed significant correlation (p ≤ 0.05) between single-pulse and burst ITD thresholds at 300, 600, and 1,000 pps (although not at 100 pps, p = 0.16) when high-threshold electrodes were included, but not at any rate when the high-threshold electrodes were excluded from the analysis. In other words, when single-pulse thresholds were very poor (in excess of 1 ms), sensitivity with longer bursts was also very poor, but otherwise, ITD performance with the two stimuli was not correlated. High-rate burst thresholds also appear not readily predictable from low-rate thresholds. This is indicated by the lack of significant (Pearson) correlation between thresholds at 100 and 1,000 Hz when including data for modulated and unmodulated signals for which thresholds were measurable at both rates. In agreement with “Effect of pulse rate and modulation rate at three places,” the results of the ANCOVA showed a highly significant effect of rate irrespective of whether electrode pairs with single-pulse thresholds in excess of 1 ms were included [F(3,54) = 21.4, p < 0.001] or not [F(3,46) = 22.3, p < 0.001]. The effect of place was again not significant, but showed a stronger trend when the electrodes with high single-pulse thresholds were included than when they were not.
Discussion
Overall, electrical ITD thresholds were similar for apical, mid, and basal stimulation and increased at all three places as pulse rate or modulation rate increased from 100 to 1,000 Hz. Listeners with normal hearing show very different effects of rate for high-frequency transposed tones and low-frequency pure tones (see “Introduction”), which might be due to differences in peripheral responses with the two signals, but could also reflect fundamentally different ITD processing for the two regions. The data presented here suggest that the latter is unlikely because thresholds were similar for high- and low-frequency place of stimulation at all rates tested when peripheral processes were better matched than is possible with acoustic stimulation. There was only a slight increase in thresholds (~15%) for the most basal place of stimulation, which may be attributed to poorer neural survival in that region, although a comparable effect has also been reported in some studies with normal-hearing listeners attending envelope ITD cues (e.g., Bernstein and Trahiotis 1994). The minimal effect of place for the electrical stimuli is not likely due to insufficient insertion depth of the most apical bands to ensure activation of regions normally associated with low-frequency hearing (see “Introduction”). Even if in some cases insertions were less deep than average, the fairly broad current spread associated with electrical stimulation will have extended to that depth.
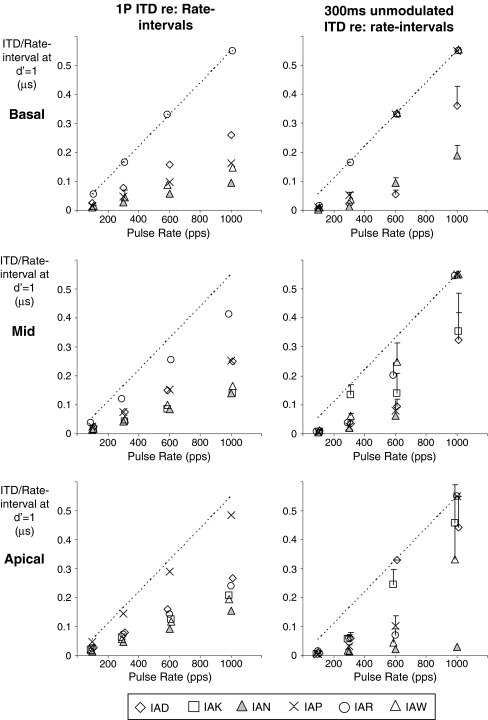
One contribution to the limited range of rates over which electrical ITDs are heard will be refractory behavior of the auditory nerve at rates beyond a few hundred Hz and at higher rates also increased uncertainty in firing times due to latency shifts (e.g., Javel and Shepherd 2000). In addition, as rates increase, cue ambiguity is introduced as the ongoing ITD represents an increasing proportion of the IPI. To illustrate, Figure 4 shows ITD thresholds for single-pulse stimuli (left column) and 300-ms unmodulated bursts (right column) from Figure 1, replotted as a fraction of the interpulse interval for the rates used in this study (akin to interaural phase delay for sinusoidal stimulation). Note that values in Figure 4 are halved relative to those in Figure 1 to show actual ITDs contained in left- or right-ear-leading signals. Considered in relation to the IPI at 1,000 pps, single-pulse threshold ITDs around 200 μs correspond to about 0.2 times the IPI. If that ITD is applied repeatedly to each pulse in a 300-ms burst, the ear that leads by 0.2 times the IPI also lags by 0.8 times that interval when a pulse in the leading ear is paired with the previous pulse in the lagging ear. Because the resultant conflicting ITD of −800 μs approaches that available from natural head width delays, it may activate a significant number of ITD-sensitive neurons and therefore substantially influence lateralization. At 600 pps, the same process results in conflicting delays of almost −1,500 μs. Although that value considerably exceeds natural head width delays, it is possible that either broadly tuned ITD neurons or those tuned beyond the head width range so as to sharpen response variation within the range (e.g. McAlpine and Grothe 2003) might be activated and present some conflicting cues. When rates are as low as 300 pps, the conflicting ITD is about 3 ms, which is probably large enough to preclude any significant response. The reduction of ITD sensitivity at a rate as low as 300 Hz compared to 100 Hz therefore suggests that other factors may play a role, such as the synchronous activation over broad regions along the cochlea with electrical pulses in contrast to the systematic phase shifts normally encountered over those regions in association with traveling wave delays for low frequencies in normal hearing. Colburn et al. (2008) have recently presented a model of brainstem responses to bilateral electrical stimulation which shows how high synchronization of the neural responses to electrical stimuli can lead to saturated rate ITD functions with poor ITD discrimination and that increased phase dispersion of the response can substantially improve that performance for average rates of a few hundred hertz.
FIG. 4.
ITD threshold data from Figure 1 replotted as a fraction of the interpulse interval for rates between 100 and 1,000 pps. Thresholds for single-pulse stimuli are shown in the left column and for 300-ms unmodulated bursts in the right column. The top, middle, and apical rows in each column again show results for basal, mid, and apical electrode pairs, respectively, and individual subject data are shown by the different symbols. Unmeasurable thresholds or those in excess of 1 ms that reflect an inability to discriminate left from right ear delays when cues in each ear were 500 μs or more are shown along the dashed diagonal line representing a threshold of 1100 μs.
Although these BiCI listeners were selected for good sensitivity at low rates, the results showed considerable intersubject variation at higher rates. Part of that variation may be due to asymmetries in the neural responses in the two ears—even when overall loudness and place pitch are optimally matched, the number of units firing, their spatial distribution, and distribution of thresholds are likely to differ. Such differences will lead to different refractory effects in the two ears and therefore increase dichotic firing as rates increase. Subject IAN displayed unusually good high-rate ITD sensitivity particularly on the most apical electrode (Fig. 1, bottom row, filled triangles). The fact that performance did not deteriorate over the entire range between 100 and 1000 Hz differs from any other BiCI user tested to date by the present authors. Unfortunately, that subject has not been available for further tests to investigate this unusual result to exclude, for example, an explanation based on electrophonic activation of residual hearing structures. If that subject’s results are strictly due to electrical activation of the nerves, it suggests minimal impact of dichotic neural firing even at 1,000 pps in this listener. Perhaps that could be explained by fortuitously symmetric distributions of surviving neurons with well-matched thresholds across ears. In addition, the listener’s ability to hear smaller onset cues, as evidenced by having the lowest single-pulse thresholds, meant that smaller ITDs could be used, leading to reduced ongoing cue ambiguity in relation to the IPI at high rates. The two best performers, when tested at 600 Hz, maintained similar ITD sensitivity with either fast or slow (10 ms) onset ramps. While it is possible that the slow ramp did not fully eliminate the onset cue for those listeners, the findings that (a) sensitivity with ramped stimuli was better than with single pulses and (b) single-pulse thresholds did not predict fast-rise-time burst thresholds at 600 pps when electrodes with thresholds exceeding 1 ms were excluded indicate that ongoing cues played a significant role at 600 pps in those subjects. That conclusion is in agreement with the observer weighting data from van Hoesel (2008) which showed that although post-onset ITD weighting was much reduced at 600 pps compared to 100 pps, it remained positive for several subjects. It is also in agreement with the data from Laback et al. (2007) which showed that one of the four listeners tested with brief four-pulse stimuli was able to hear ITD cues applied only to the second and third pulses at 800 pps.
The finding that electrical ITD sensitivity for 300-ms bursts at 1,000 pps was for most listeners actually worse than for a single pulse implies that rather than just adding no information, the inclusion of post-onset cues can actually degrade overall performance at that rate. That may be explained by the consideration that the single pulse presents a single unambiguous cue, whereas the 300-ms burst contains ongoing cue ambiguity in relation to the IPI, as discussed above, as well as strong refractory effects leading to unpredictable pairing of pulses across the ears. Note that for ITD ambiguity in relation to the IPI to play a role at high rates, listeners need to be sensitive to ITDs at those actual rates, whereas refractory effects can reduce neural firing rates and introduce low-rate ITD cues contained in binaural pulses that may or may not be appropriately paired across the ears. It seems less likely that the onset cue itself was degraded at 1,000 pps through temporal smearing of neural responses to the early pulses in the burst. Evidence to that effect was presented in van Hoesel (2007) in which a two-pulse precedence experiment at 1,000 pps showed a large asymmetry in BiCI listeners’ abilities to hear ITDs depending on whether they were applied to the lead or lag pulse. That result would not be expected if smearing substantially degraded the onset cue salience at 1,000 pps. Direct onset cue degradation at high rates is also not supported by the data in Figure 2, which show that listeners with reduced ITD sensitivity resulting from longer rise times demonstrated the same effect with both unmodulated and modulated signals. That finding implies that onsets provided strong cues with either signal when using the fast rise time despite the much higher pulse rate in the modulated signal.
Increases in modulation rate elevated ITD thresholds in a manner similar to that seen with pulse rate increases. While subject-averaged thresholds were higher for the modulated than unmodulated signals at all rates, pairwise comparisons at each rate showed only the difference at 300 Hz to be significant. In the study by van Hoesel (2007), a similar difference was observed at a modulation rate of 300 Hz and not at 100 Hz. The poorer performance with modulated than unmodulated signals may be due to the use of lower stimulation levels to maintain comparable loudness (van Hoesel 2007). It may also be due to the shortened interval in each modulation cycle during which nerves can recover from high stimulation levels when compared to the temporally succinct single-pulse per cycle condition, which may result in greater firing time uncertainty. That may account for why the greatest difference was seen at 300 Hz. At the lower rate of 100 Hz, even the modulated signal affords the nerves sufficient recovery time to allow them to fire at the onset of the next high-level segment of the modulation cycle and at higher rates (600 and 1,000 Hz) even the single-pulse per cycle stimulus presents insufficient recovery times.
Recently, Laback and Majdak (2008) reported improved ITD sensitivity at high rates when they applied diotic temporal jitter and attributed that result to “restarting” of the binaurally adapted auditory system (e.g., Hafter and Buell 1990). However, as discussed in the observer weighting study by van Hoesel (2008), that explanation is unable to account for why the jitter improved performance at 800 pps and higher, but not at 400 pps despite evidence of strong onset dominance at the latter rate in BiCI users. An alternative explanation supported by the data from that observer weighting study is that the effect of jitter was due to the introduction of confounding low rate cues and reduced ambiguity rather than any actual improvement in high-rate ITD sensitivity per se. Although it may be of some interest to determine more accurately the high-rate ITD thresholds that in the present study often exceeded 1 ms, it would be of limited practical value if they are beyond the natural head width range. Poor ITD performance at high pulse rates may largely account for why the benefits derived from fine-timing ITDs have been minimal for BiCI users to date even when using sound-coding strategies that specifically preserve that cue (van Hoesel et al. 2008). In everyday listening with clinical processors that preserve only envelope timing cues, the presence of multiple sound sources is likely to increase envelope fluctuation rates, which will degrade ITD salience as shown by the present results with modulated signals. Even at modest rates, ITD sensitivity with multichannel processors can be compromised by out-of-phase stimulation on nearby electrodes that lead to effective increases in stimulation rate as seen by the nerve (Jones et al. 2008).
Conclusions
Overall, electrical ITD sensitivity was similar for all three places of stimulation along the cochlea and decreased as a function of pulse rate or modulation rate between 100 and 1,000 Hz. That similarity across places is in agreement with a common ITD processing mechanism for both high- and low-frequency regions and is largely peripherally mediated with regards to the effect of rate. Although all six BiCI users showed good ITD sensitivity at the lowest rate, considerable intersubject variation was observed as rates increased, with one exceptional listener showing no effect of rate up to 1,000 Hz for the most apical electrode pair. Compared to single-pulse thresholds, the addition of ongoing cues in the 300-ms bursts generally improved performance at 100 and 300 Hz, had no effect at 600 Hz, and actually reduced overall performance at 1,000 Hz. The reduction at the highest rate may be explained by substantially increased cue ambiguity in relation to the IPI, which falls within the physiologically relevant range at that rate, as well as dichotic neural firing due to refractory effects. The latter may also explain why thresholds are higher for high-rate modulated signals than for unmodulated pulse trains. The degrading effect of rate increases on ITD sensitivity in these bilateral CI users is consistent with that observed in listeners with normal hearing attending high-frequency envelope cues rather than low-frequency fine timing cues.
Acknowledgments
The authors gratefully acknowledge participation of the bilateral implant users in this study. Financial support for the work was provided by the NIH-NIDCD (R01 DC 003083 to Ruth Litovsky) and for the first author by The Hearing CRC, Australia. Some of the data described were presented at CIAP 2005 (Jones et al., 2005) and at the Association for Research in Otolaryngology Midwinter Meeting 2006 (Jones et al., 2006).
References
- Bernstein LR, Trahiotis C. Detection of interaural delay in high-frequency SAM tones, two-tone complexes, and bands of noise. J. Acoust. Soc. Am. 95:3561–3567, 1994. [DOI] [PubMed]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using transposed stimuli. J. Acoust. Soc. Am. 112(3):1026–1036, 2002. [DOI] [PubMed]
- Colurn HS, Equissaud P. An auditory-nerve model for interaural time discrimination of high-frequency complex stimuli. J. Acoust. Soc. Am. Suppl. 159:523, 1976.
- Colburn HS, Chung Y, Zhou Y, Brughera A. Models of brainstem responses to bilateral electrical stimulation. JARO, 10:91–110, 2008. [DOI] [PMC free article] [PubMed]
- Foster DH, Bischof WF. Thresholds from psychometric functions: superiority of bootstrap to incremental and probit variance estimators. Psychol. Bull. 109:152–159, 1991. [DOI]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York, Wiley, 1966.
- Grantham DW, Ashmead DH, Ricketts TA, Labadie RF, Haynes DS. Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants. Ear Hear. 28:524–41, 2007. [DOI] [PubMed]
- Hafter ER, Buell TN. Restarting the adapted binaural system. J. Acoust. Soc. Am 88:806–812, 1990. [DOI] [PubMed]
- Hafter ER, Dye RH, Jr. Detection of interaural differences of time in trains of high-frequency clicks as a function of interclick interval and number. J. Acoust. Soc. Am 73(5):1708–1713, 1983. [DOI] [PubMed]
- Javel E, Shepherd RK. Electrical stimulation of the auditory nerve. III. Response initiation sites and temporal fine structure. Hear. Res. 140:45–76, 2000. [DOI] [PubMed]
- Jones GL, Litovsky RY, Agrawal SS, van Hoesel RJM. Effect of Stimulation Rate and Interaural Electrode Pairing on ITD Sensitivity in Bilateral Cochlear Implant Users, Conference on Implantable Auditory Prostheses, Asilomar, USA, July 2005.
- Jones GL, Litovsky RY, Agrawal SS, van Hoesel RJM. “Effect of Stimulation Rate and Modulation Rate on ITD Sensitivity in Bilateral Cochlear Implant Users”, Association for Research in Otolaryngology, 29th Midwinter Meeting, 2006. [DOI] [PMC free article] [PubMed]
- Jones G, van Hoesel R, Litovsky R. Effect of channel interactions on sensitivity to binaural timing cues in electrical hearing. J. Acoust. Soc. Am. 123(5):3055, 2008. [DOI]
- Klumpp RG, Eady HR. Some measurements of interaural time difference thresholds. J. Acoust. Soc. Am. 28:859–860, 1956. [DOI]
- Laback B, Majdak P. Binaural jitter improves interaural time-difference sensitivity of cochlear implantees at high pulse rates. PNAS 105(2):814–817, 2008. [DOI] [PMC free article] [PubMed]
- Laback B, Majdak P, Baumgartner W-D. Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing. J. Acoust. Soc. Am 121:2182–2192, 2007. [DOI] [PubMed]
- Levitt H, Rabiner LR. Predicting binaural gain in intelligibility and release form masking for speech. J. Acoust. Soc. Am. 42:820–829, 1962. [DOI] [PubMed]
- Litovsky RY, Agrawal SS, Jones GJ, Henry B, Van Hoesel RJM. Effect of interaural electrode pairing on binaural sensitivity in bilateral cochlear implant users. Association for Research in Otolaryngology, 28th Midwinter Meeting, 2005.
- Litovsky RY, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study. Ear Hear. 27:714–731, 2006. [DOI] [PMC free article] [PubMed]
- MacMillan NA, Creelman CD. Detection Theory, A User’s Guide, 2nd Ed. Lawrence Erlbaum Associates, Inc., New Jersey, USA, 2005. ISBN 0-8058-4231-4.
- Majdak P, Laback B, Baumgartner W-D. Effects of interaural time differences in fine-structure and envelope on lateral discrimination in electric hearing. J. Acoust. Soc. Am. 120:2190–2201, 2006. [DOI] [PubMed]
- McAlpine D, Grothe B. Sound localization and delay lines—Do mammals fit the model? Trends NeuroSci. 26:347–350, 2003. [DOI] [PubMed]
- Neumann AC, Haravon A, Sislian N, Waltzman SB. Sound-direction identification with bilateral cochlear implants. Ear Hear. 28:73–82, 2007. [DOI] [PubMed]
- Nopp P, Schleich P, D'Haese P. Sound localization in bilateral users of Med-El Combi 40/40+ cochlear implants. Ear Hear. 25:205–214, 2004. [DOI] [PubMed]
- Oxenham AJ, Bernstein JGW, Penagos H. Correct tonotopic representation is necessary for complex pitch perception. PNAS. 101:1421–1425, 2004. [DOI] [PMC free article] [PubMed]
- Saberi K. Observer weighting of interaural delays in filtered impulses. Percept. Psychophys. 58:1037–1046, 1996. [DOI] [PubMed]
- Stakhovskaya O, Sridar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. JARO. 8:220–233, 2007. [DOI] [PMC free article] [PubMed]
- Stecker GC, Hafter ER. Temporal weighting in sound localization. J. Acoust. Soc. Am. 112:1046–1057, 2002. [DOI] [PMC free article] [PubMed]
- van de Par S, Kohlrausch A. A new approach to comparing binaural masking level differences at low and high frequencies. J. Acoust. Soc. Am. 101:1671–1680, 1997. [DOI] [PubMed]
- van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiol. Neurootol 9:234–246, 2004. [DOI] [PubMed]
- van Hoesel RJM. Sensitivity to binaural timing in bilateral cochlear implant users. J. Acoust. Soc. Am. 121:2192–2206, 2007. [DOI] [PubMed]
- van Hoesel RJM. Observer weighting of level and timing cues in bilateral cochlear implant users. J. Acoust. Soc. Am. 124:3861–3872, 2008. [DOI] [PubMed]
- van Hoesel RJM, Tyler RS. Speech perception and localization with bilateral cochlear implants. J. Acoust. Soc. Am. 113:1617–1630, 2003. [DOI] [PubMed]
- van Hoesel R, Böhm M, Pesch J, Vandali A, Battmer RD, Lenarz T. Binaural speech unmasking and localization in noise with bilateral cochlear implants using envelope and fine-timing based strategies. J. Acoust. Soc. Am. 123:2249–2263, 2008. [DOI] [PubMed]
- Wightman F, Kistler DJ. The dominant role of low frequency interaural time differences in sound localization. J. Acoust. Soc. Am. 91:1648–1661, 1992. [DOI] [PubMed]