Abstract
Normal-hearing (NH) listeners and hearing-impaired (HI) listeners detected and discriminated time-reversed harmonic complexes constructed of equal-amplitude harmonic components with fundamental frequencies (F0s) ranging from 50 to 800 Hz. Component starting phases were selected according to the positive and negative Schroeder-phase algorithms to produce within-period frequency sweeps with relatively flat temporal envelopes. Detection thresholds were not affected by component starting phases for either group of listeners. At presentation levels of 80 dB SPL, NH listeners could discriminate the two waveforms nearly perfectly when the F0s were less than 300–400 Hz but fell to chance performance for higher F0s. HI listeners performed significantly poorer, with reduced discrimination at several of the F0s. In contrast, at a lower presentation level meant to nearly equate sensation levels for the two groups, NH listeners’ discrimination was poorer than HI listeners at most F0s. Roving presentation levels had little effect on performance by NH listeners but reduced performance by HI listeners. The differential impact of roving level suggests a weaker perception of timbre differences and a greater susceptibility to the detrimental effects of experimental uncertainty in HI listeners.
Keywords: harmonic complex, hearing impairment, Schroeder phase, stimulus uncertainty, frequency sweep
Introduction
Several studies have demonstrated differences in the masking effectiveness of positive- (+SCHR) and negative-phase (−SCHR) versions of Schroeder harmonic complexes in normal-hearing (NH) and hearing-impaired (HI) listeners (Carlyon and Datta 1997; Kohlrausch and Sander 1995; Lauer et al. 2006; Leek et al. 2000; Lentz and Leek 2001; Oxenham and Dau 2001, 2004; Smith et al. 1986; Summers and Leek 1998). These complexes have similar envelopes and long-term frequency spectra but reversed within-period temporal structure. While +SCHR often produce less masking than −SCHR in NH listeners, the difference is reduced in HI listeners. The difference in masking between NH and HI listeners is thought to be related to the loss of nonlinear active processing mechanisms with cochlear damage.
Although the +SCHR and –SCHR have nearly identical temporal envelopes, the phase characteristics of these complexes result in a systematic interaction with the traveling wave that produces quite different envelopes after cochlear processing in a normal cochlea. Basilar membrane responses to +SCHR are more peaked and contain less spectral energy than responses to –SCHR in normal chinchilla ears, while damaged ears show more similar responses to the two waveforms (Recio and Rhode 2000). Similar findings were reported in guinea pigs (Summers et al. 2003). In NH humans, acoustic reflex thresholds are lower for −SCHR than for +SCHR (Kubli et al. 2005), and HI listeners perceive much smaller differences in loudness between +SCHR and −SCHR compared to NH listeners (Mauermann and Hohmann 2007). These findings are consistent with a greater magnitude of internal stimulation in the normal auditory system occurring in response to the −SCHR.
Perceptual differences between +SCHR and –SCHR must result from distinctive outputs of the auditory filters, requiring that the fundamental frequencies (F0s) of the Schroeder-phase complexes are sufficiently low so that several harmonic components are processed within at least some of the auditory filters. A previous study showed that NH listeners can discriminate between +SCHR and −SCHR with F0s up to 300 to 400 Hz (Dooling et al. 2002). Cochlear-implant users can discriminate the +SCHR and –SCHR at better than chance performance for F0s of 400 Hz and less, but they did not perform as well as NH subjects (Drennan et al., 2008). Discrimination of +SCHR and −SCHR has not been tested in HI non-cochlear implant listeners; however, HI canaries have been shown to perform slightly better than NH canaries when discriminating between +SCHR and −SCHR (Lauer et al. 2007). The difference in discrimination ability in NH and HI canaries was attributed to differences in frequency selectivity, in that broader filters in damaged ears may support better resolution of temporal characteristics of sounds.
Four experiments explored detection and discrimination of +SCHR and −SCHR by NH and HI listeners. First, thresholds were determined to identify potential audibility differences resulting from differing internal waveform shapes. Next, the ability of the two listener groups to discriminate between +SCHR and −SCHR for a range of F0s was compared at equal sound pressure levels (SPL) and equal sensation levels (SL). Finally, the potential roles of loudness and stimulus uncertainty on discrimination performance were evaluated by roving the stimulus level on each presentation. These conditions are outlined in Table 1.
TABLE 1.
Summary table of four experiments carried out in this study
| Experiment | Population | Stimulus Level (dB SPL) | Rove/No rove | F0 tested (Hz) |
|---|---|---|---|---|
| Experiment 1 | NH, HI | Detection threshold | Not applicable | 50–800 |
| Experiment 2 | NH, HI | 80 | No rove | 50–800 |
| Experiment 3 | NH | 40 | No rove | 50–500 |
| Experiment 4 | NH, HI | 80 | Rove | 50–800 |
Experiment 1 is a detection experiment, while the remaining experiments test discriminations between positive and negative Schroeder-phase waveforms
Materials and methods
Participants
Six NH listeners (age 27–73 years; mean = 49.17, SD = 17.42) and six HI listeners (age 65–81 years; mean = 73.17, SD = 5.23) participated in the experiments. All participants provided written informed consent at the time of their enrollment in the study. Audiograms were obtained for all listeners. All NH listeners had pure tone thresholds within 25 dB of the ANSI (2004) standards between 250 and 4,000 Hz, except for NH6, the oldest NH listener. Listener NH6 had thresholds within 25 dB of the ANSI (2004) standards between 500 and 4,000 Hz but showed mild hearing loss at 250 Hz. HI listeners had flat to gradually sloping mild to moderate bilateral hearing losses between 250 and 4,000 Hz. The hearing losses were presumed to be sensorineural based on air- and bone-conduction measures. The right ear was tested for all NH listeners. The ear with lower audiometric thresholds or a flatter audiometric configuration was tested in HI listeners. The audiograms and ages of the listeners are listed in Table 2. All listeners except one (HI6) had prior experience with psychoacoustic measurements. Listeners NH1 and NH4 were the first and third authors, respectively. All procedures were approved by the Institutional Review Boards at the University of Maryland and Walter Reed Army Medical Center.
TABLE 2.
Audiometric data for normal-hearing and hearing-impaired listeners
| Listener | Age | 250 | 500 | 1000 | 1500 | 2000 | 3000 | 4000 | 6000 | 8000 (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| NH1 | 27 | 10 | 10 | 5 | – | 10 | 15 | 10 | 5 | 10 |
| NH2 | 47 | 10 | 10 | 10 | 5 | 15 | 10 | 10 | 10 | 0 |
| NH3 | 32 | 10 | 5 | 5 | – | 5 | −5 | 0 | −5 | −5 |
| NH4 | 57 | 15 | 15 | 15 | 15 | 5 | 5 | 10 | 5 | 20 |
| NH5 | 59 | 0 | 0 | 5 | 10 | 10 | 10 | 5 | 20 | 20 |
| NH6 | 73 | 35 | 20 | 10 | – | 25 | 25 | 15 | 10 | 25 |
| HI1 | 65 | 35 | 40 | 30 | 30 | 30 | 30 | 40 | 35 | 30 |
| HI2 | 81 | 40 | 40 | 60 | 55 | 60 | 65 | 70 | 70 | 65 |
| HI3 | 71 | 35 | 40 | 45 | 35 | 35 | 45 | 40 | 30 | 35 |
| HI4 | 74 | 35 | 40 | 40 | – | 30 | 30 | 35 | 45 | 40 |
| HI5 | 71 | 40 | 40 | 35 | 35 | 45 | 45 | 40 | 45 | 50 |
| HI6 | 73 | 25 | 35 | 35 | 35 | 40 | 45 | 60 | 65 | 60 |
Thresholds are reported in dB HL (ANSI 2004)
Stimuli
Harmonic complexes were constructed with component starting phases, θn, selected according to an algorithm developed by Schroeder (1970) designed to produce complexes with very flat temporal envelopes:
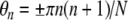 |
1 |
where n is the component number and N is the total number of harmonic components in the complex. When the Schroeder equation is positive, phases increase with harmonic number, and when it is negative, phases decrease. The resulting waveforms are the temporal reverse of one another, with monotonically decreasing (+SCHR) or increasing (−SCHR) frequencies within each period. If the F0 is low enough (i.e., the fundamental period is long enough in duration), the two waveforms may be perceived as different. However, if the within-period “sweep” occurs too rapidly, the two sound identical.
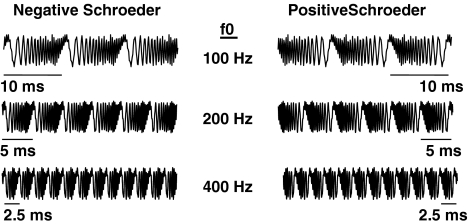
+SCHR and −SCHR complexes with F0s of 50, 100, 150, 200, 300, 400, 500, 600, and 800 Hz were created by summing equal-amplitude harmonics between 200 and 5,000 Hz, unless the F0 was greater than 200 Hz. In this case, harmonics between the F0 and 5,000 Hz were summed. The periods of these stimuli ranged from 20 to 1.25 ms, where complexes with higher F0s had shorter periods. The waveforms were 260 ms in duration, with 20 ms cos2 onset/offset ramps. Figure 1 shows examples of the negative- and positive-phase waveforms for several of the F0s. Only 30 ms of each example are shown so that the temporal fine structure can be observed.
FIG. 1.
Examples of −SCHR (left column) and +SCHR complexes for three of the F0s used in this study. Only 30 ms of the stimuli are shown here. The full stimuli used in the experiments were all 260 ms in duration.
The stimuli were generated digitally offline using MATLAB and stored on a PC. Tucker Davis Technologies (TDT) System 2 programmable modules were used to present the stimuli to the listeners in a sound-treated booth. Stored sound files were played from a 16-bit digital-to-analog converter (TDT DA1) at a sampling rate of 40 kHz, attenuated with a programmable attenuator (TDT PA4), and fed through a headphone buffer (TDT HB6) into one phone of a TDH-49 headset. Stimuli were calibrated periodically throughout the experiment to verify sound levels using a Larson-Davis sound level meter and headphone coupler (System 824, AEC100 NBS 9A).
Experiment 1: detection of Schroeder-phase complexes
It is important to establish whether the differences in cochlear excitation produced by +SCHR and –SCHR result in differences in detectability in NH listeners, but not in HI listeners, since sensation level differences between the two waveforms could aid discrimination. Absolute thresholds for +SCHR and −SCHR were measured for each F0 to determine if they were equally detectable and to calculate sensation levels for the experiments to follow.
Thresholds for +SCHR and −SCHR with different F0s were measured in a two-alternative forced choice task, with trial-by-trial signal levels chosen according to a three-down one-up adaptive tracking method to estimate a threshold of 79.4% correct detection (Levitt 1971). On each trial, lights on a button box indicated two intervals separated by 300 ms. A stimulus was presented randomly in one of the two intervals, and listeners were asked to identify which interval coincided with the sound by pressing one of two buttons marked with “1” or “2.”
At the beginning of a threshold track, the stimulus level was set to about 30 dB above the presumed threshold. The initial step size was 5 dB, and after three reversals, the step size was reduced to 2 dB. Each track consisted of nine reversals, and the average of the last six was taken as threshold. Listeners ran two tracks for each F0: one with +SCHR and one with −SCHR. Stimuli were tested in random order.
Experiments 2–4: discrimination of Schroeder-phase harmonic complexes
NH and HI listeners’ ability to discriminate between +SCHR and −SCHR was measured at equal SPLs and approximately equal SLs for a series of F0s. For equal SPL tests (Experiment 2), a presentation level of 80 dB SPL was used so that the stimuli were clearly audible to all HI listeners. The 80-dB SPL presentation level corresponds to individual SLs of about 15–40 dB for HI listeners and 50–65 dB for NH listeners. Leek et al. (2000) showed smaller differences in masking produced by +SCHR and –SCHR complexes presented at 40 dB SPL than at higher presentation levels in NH listeners, suggesting that the internal waveform shapes might be more similar at lower levels. To determine whether differences in discrimination between NH and HI listeners were the result of the lower SL for the latter group, NH listeners were also tested at 40 dB SPL for F0s of 50 to 500 Hz in Experiment 3, so that NH and HI listeners could be compared at approximately equal SLs. Finally, both groups of listeners were evaluated again at levels roving plus or minus 5 dB around 80 dB SPL to reduce the effectiveness of possible loudness cues (Experiment 4).
Percent correct discriminations between +SCHR and –SCHR harmonic complexes were measured using a standard/two-alternative forced choice task. On each trial, listeners heard either the +SCHR or the –SCHR as a standard sound in the first interval followed by two presentations of comparison stimuli, one being the standard again and one the opposite-sign complex. The F0 was the same on all three presentations within a trial. The order of presentation (+SCHR or –SCHR) in intervals two and three was random. An interstimulus interval of 300 ms separated the three sounds. Listeners were asked to identify the stimulus that was different from the standard by pressing one of two buttons labeled “1” and “2.” Correct answer feedback was provided to the listener following each response. The standard stimulus (positive or negative) remained constant on each trial within a block of 50 trials, as did the F0.
Two blocks of 50 trials were run for each F0: one with the +SCHR as the standard and one with the –SCHR as the standard. Listeners began with the 50-Hz F0 comparisons (presumed to be the easiest because the fundamental period is longest) to familiarize them with the task. The standard stimulus (+SCHR or –SCHR) and the F0 of the standard stimulus were then chosen randomly for each block of trials for the remaining F0s, selected in random order for each listener. Thus, listeners were tested on a total of 100 trials for each F0. Percent correct is reported as the average of the two blocks.
Statistical analyses
Statistical methods used to analyze the data are described here. Application of these methods to identify significant effects in the data will be described in Section 3 (Results).
Experiment 1 (Detection) A 2 × 2 × 9 (phase×group×F0) mixed factor analysis of variance was performed on the detection data to identify effects of hearing status and F0.
Experiments 2–4 (Discrimination) Rather than analyze the data from each experiment separately, a linear mixed model (Fitzmaurice et al. 2004) was fit to the data from the three discrimination experiments. Specific contrasts from the fitted model were used to test the hypotheses of this study. The model included categorical main effects of hearing status (HI versus normal hearing), stimulus level, rove condition (no level rove versus roving level), and F0. Two-way interactions tested in the model were between hearing status and F0, hearing status and rove/no rove presentation conditions, F0 and rove/no rove conditions, and stimulus level and F0. Although the three-way interaction between hearing status, F0, and rove condition was included in the model, it was not significant at the 0.05 test level [F(8,106) = 1.25, p = 0.28] so this interaction was omitted in subsequent individual contrast analyses. A subject-level random intercept was also included in the model, allowing some subjects to have better or worse performance on the tests. The goodness-of-fit analysis of the linear mixed model indicated no gross deviations of the fitted model to the data, but there was considerable variability in the residuals among F0s. This heteroscedasticity is not unexpected, given the ceiling (100%) and floor (chance = 50%) limitations on the data. The heteroscedasticity was resolved by including a log-linear model of the variance in the general linear model with F0 as the predictor (Aitken 1987). This method stabilized the variance in the residuals across F0s.
Table 3 outlines the contrasts that were tested combining data from selected experiments. For each contrast identified, the null hypothesis was that the average difference in percent correct discrimination across tested F0s was not significantly different from zero. For each contrast evaluated, differences in performance for the individual F0s were also tested. These were derived directly from linear combinations of the estimated parameters of the linear mixed model. In all, 42 contrasts were tested across the three discrimination experiments, giving rise to concerns of multiplicity of tests. In the analyses, therefore, the p values were adjusted using False Discovery Rate controls so that the proportion of Type 1 errors among all rejected null hypotheses is not greater than 0.05 (Benjamini and Hochberg 1995; Verhoeven et al. 2005). This method controls the accuracy over all the tests, while preserving the power to detect important differences that might be lost using the less powerful, but more commonly used, Bonferroni p value adjustments.
TABLE 3.
Contrasts evaluated using the linear model
| Contrast | Data From | Stimulus Level (dB SPL) | Rove/no rove | F0 tested (Hz) | Data shown |
|---|---|---|---|---|---|
| NH vs HI, equal SPL | Exp 2 | 80 | No rove | 50–800 | Figure 3A |
| NH vs HI, equal SL | Exp 2, 3 | 40 (NH),80 (HI) | No rove | 50–500 | Figure 3B |
| NH low vs high level | Exp 2, 3 | 40, 80 | No rove | 50–500 | Not shown |
| NH rove vs no rove | Exp 2,4 | 80 | Rove, no rove | 50–800 | Figure 4A |
| NH vs HI, rove | Exp 4 | 80 | Rove | 50–800 | Not shown |
| HI rove vs no rove | Exp 2, 4 | 80 | Rove, no rove | 50–800 | Figure 4B |
Each contrast tests the null hypothesis that the average difference in performance across fundamental frequencies is not significantly different from 0. The last column indicates which figure displays the data from each contrast
As data from each experiment are presented, the overall statistical results of the main effects and interactions of the linear mixed model are reported, along with pairwise contrasts over the range of F0s.
Results
Detection of Schroeder-phase complexes
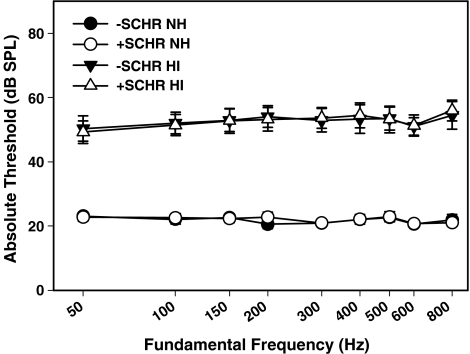
Absolute thresholds for +SCHR and −SCHR with different F0s, along with standard errors, are shown in Figure 2 for NH and HI listeners. Average thresholds for NH listeners were near 20 dB SPL for all stimuli. Average thresholds in HI listeners were 50 dB SPL or higher for all stimuli. There was more variation in thresholds for HI than for NH listeners. Individual thresholds ranged from 15 to 29 dB SPL for NH listeners and from 40 to 66 dB SPL for HI listeners. There were no significant differences overall between thresholds for +SCHR and thresholds for −SCHR or thresholds as a function of F0; however, there was a significant difference in thresholds between NH and HI listeners [F(1, 10) = 80.99, p < 0.0001]. There was a significant interaction of F0 and listener group [F(8, 80) = 2.59, p = 0.014], which probably reflects the slight rise in thresholds with F0 in the HI group.
FIG. 2.
Absolute thresholds for +SCHR and −SCHR with different F0s in normal-hearing (circles) and hearing-impaired listeners (triangles).
Discrimination of Schroeder-phase harmonic complexes
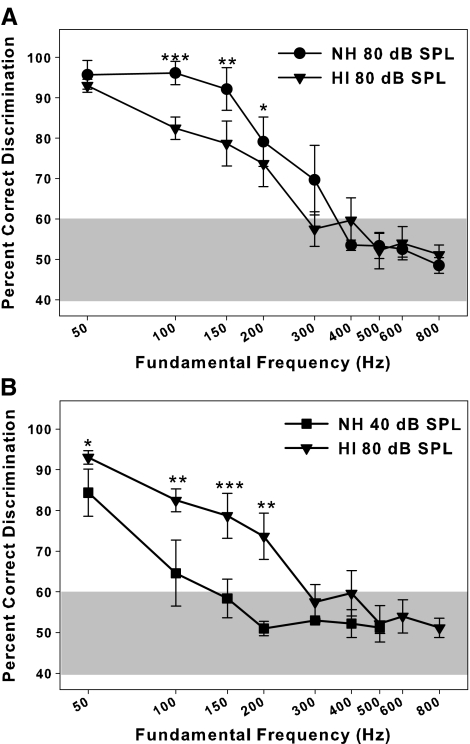
Percent correct discriminations for NH and HI listeners tested at 80 dB SPL are shown in Figure 3A. Error bars indicate standard errors. Any points falling above 60% correct represent performance that is significantly different from chance at the 0.05 level of probability. NH listeners showed near perfect discrimination at F0s up to 150 Hz, but performance dropped for stimuli with F0s of 200–300 Hz and decreased to chance at 400 Hz. These results in NH listeners are in good agreement with those reported by Dooling et al. (2002) for discrimination of similar harmonic stimuli by NH humans. HI listeners showed good discrimination for a F0 of 50 Hz, but performance deteriorated for higher F0s. Performance by HI listeners fell to chance levels for F0s of 300 Hz and above. The average difference between the two groups was significant [t(21.3) = 3.12, p = .01], indicating that, at 80 dB SPL, overall performance was significantly better for the NH than the HI group. F0s that showed differences between the two groups were 100, 150, and 200 Hz (all p < .05). No significant differences were observed for higher F0s (p > .08) or for a F0 of 50 Hz (p = 0.06).
FIG. 3.
Percent correct discrimination between +SCHR and −SCHR with different F0s for normal-hearing and hearing-impaired listeners tested at equal sound pressure levels (A) and approximately equal sensation levels (B). *p < 0.05, **p < 0.01, ***p < 0.001.
Percent correct discriminations for NH listeners tested at 40 dB SPL are shown in Figure 3B along with data replotted for HI listeners tested at 80 dB SPL. Error bars indicate standard errors. This comparison shows performance of the two groups at roughly equivalent SLs. Overall, performance for NH listeners was worse than their performance at the higher level and was also worse than that of HI listeners at this approximately equal SL. The average difference between the NH listeners at the two levels was 18.57 percentage points [t(345) = −10.76, p < .00001]. When the two groups are compared at equal SL, the average difference between groups was 11.55 percentage points [t(26.9) = −4.85, p = .0002]. Performance at 40 dB SPL for NH listeners dropped to chance at 150 Hz, whereas the hearing-impaired listeners were still performing above chance until the F0 reached 300 Hz. There is, then, a strong effect of level on this discrimination, and HI listeners can actually make discriminations across shorter fundamental periods (higher F0s) than NH listeners when SLs are equated.
Effects of roving level
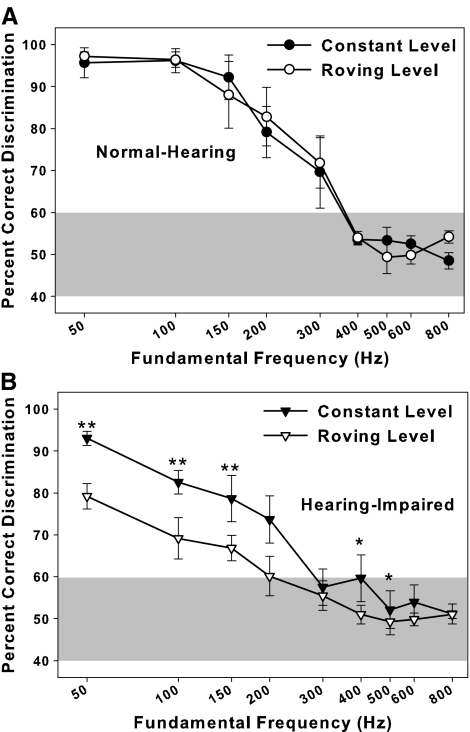
Mean percent correct discriminations for the roving level condition are plotted along with data from Experiment 2 in Figure 4 for NH (A) and HI (B) listeners. Error bars indicate standard errors. Roving the presentation level had essentially no effect on NH listeners. The average difference between conditions was 2.49 percentage points [t(442) = −1.62, p = .16]. None of the pairwise comparisons for each F0 showed significant differences (all p > .10). However, performance on the roving and fixed level conditions for the HI listeners differed significantly [average difference = 8.48, t(442) = 5.51, p < .00001], with significant pairwise differences at F0s of 50, 100, 150, 400, and 500 Hz (p < .05). Performance by HI listeners in the roving-level conditions decreased by about 10–15 percentage points for F0s of 50–200 Hz. Comparing results in the roving-level conditions across Figure 4A, B, NH listeners performed significantly better than the HI listeners [average difference = 13.01, t(21.3) = 5.79, p = .00006], and the pairwise differences were significant at p < .01 for F0s up to and including 300 Hz.
FIG. 4.
Percent correct discrimination between +SCHR and −SCHR with different F0s for normal-hearing (A) and hearing-impaired (B) listeners tested with constant and roving levels. *p < 0.05, **p < 0.01, ***p < 0.001.
To determine whether or not listeners selected responses based on relative stimulus intensities, response choices in the roving level conditions were analyzed as a function of the relative intensities of presentations on each trial. On average, NH and HI listeners did not respond to the more (or less) intense presentation within a trial. NH listeners chose the higher intensity on average 50.74% of the time, with a standard deviation across listeners of 3.67%; HI listeners chose the higher intensity presentation 49.69% of the time, with a standard deviation of 1.82%. Neither listener group selected the comparison sound that was most different in level from the level of the standard on the trial (NH: mean = 50.04%, SD = 2.08%; HI: mean = 49.16%, SD = 1.68%). This indicates that possible loudness differences between the Schroeder waveforms were not used to select responses in the roved level condition.
Discussion
Detection of Schroeder-phase complexes
Detection thresholds for +SCHR and –SCHR reported in this study (experiment 1) for NH listeners are within the range of thresholds for rising and falling frequency sweeps reported by Uppenkamp et al. (2001). As expected, HI listeners had higher thresholds for Schroeder-phase complexes than NH listeners. Neither group showed differences in thresholds between +SCHR and –SCHR. Uppenkamp et al. (2001) also found no difference in thresholds for rising versus falling sweeps. However, they did find threshold differences for sweeps with different period durations, where thresholds were slightly higher for shorter sweeps (3.1 ms) than for longer sweeps (10.5 ms). No differences across fundamental frequency were observed in NH listeners for the harmonic complexes used here. This inconsistency in results is perhaps not unexpected, given the differences in stimuli (single frequency sweeps versus harmonic complexes), stimulus duration, and level. Further, the Uppenkamp et al. (2001) stimuli were not all equal in energy. A direct comparison between the current results and those of Uppenkamp et al. (2001) is, therefore, difficult.
Despite the differences in masking effectiveness and in physiological measures reported for +SCHR and −SCHR, our results show that the two versions of the waveforms produce nearly identical absolute thresholds. Detection thresholds for +SCHR and –SCHR must be independent of the internal waveform shape of complex stimuli and must be determined by the excitation alone, without reference to phase. Preece and Wilson (1988) also reported no difference in masked thresholds for five-component waveforms with different crest factors, consistent with the suggestion that the waveform shape does not affect thresholds for harmonic complexes.
Discrimination of Schroeder-phase harmonic complexes
When stimuli were presented at equal SPLs, HI listeners could not discriminate the two Schroeder-phase waveforms as well as NH listeners. Performance at approximately equal SLs was worse for the NH listeners, and their performance dropped to chance at lower F0s than observed for HI listeners. Thus, HI listeners were actually better than NH listeners at discriminating between +SCHR and −SCHR when SL was taken into account.
Although the use of cues related to the perception of temporal fine structure are thought not to be affected by level (Moore and Sek 2009), the decreased performance by NH listeners at lower SLs is consistent with some other measures of temporal resolution (Buus and Florentine 1985; Fitzgibbons and Gordon-Salant 1987; Fitzgibbons and Wightman 1982; Florentine and Buus 1984; Glasberg et al. 1987; Irwin and Purdy 1982; Irwin et al. 1981; Tyler et al. 1982). This effect of level may be related to auditory frequency selectivity at low levels rather than to audibility. The limited range of F0s where the discrimination was possible in NH listeners may reflect the narrower auditory filters at this low level, in contrast to the broader filters of HI listeners (Glasberg and Moore 1986), as well as broader NH filters at high stimulus levels (Glasberg and Moore 1990).
Linear systems analysis predicts that poorer frequency resolution should lead to better temporal resolution due to the shorter impulse response associated with broader auditory filters (deBoer 1985; Duifhuis 1973). Following this line of reasoning, one would predict that the broadened auditory filters associated with cochlear damage would lead to better temporal resolution in HI listeners compared to NH listeners on the present task. This might occur because more components of the harmonic complex would fall within a single channel, producing a more informative within-channel waveform. Whereas at equal SPLs, both NH and HI listeners may show relatively good temporal resolution because of poor frequency resolution, the presumably narrower filters of NH listeners at 40 dB SPL could result in poorer temporal resolution. However, measures of temporal resolution in HI listeners generally have not indicated enhanced temporal processing (e.g., Grant et al. 1998; Jesteadt et al. 1976).
Another possible explanation for the decreased performance in NH listeners at a lower SL is that, well above absolute threshold, NH listeners may have additional envelope or loudness cues available to them to support the discrimination of these stimuli. The large differences in masking by +SCHR and −SCHR have been explained as a hypothesized interaction between the phase characteristic of the basilar membrane and the phase spectrum of the +SCHR waveform that produces an internally peaky within-channel waveform (Kohlrausch and Sander 1995; Leek and Lentz 2001; Oxenham and Dau 2001). This internal transformation of the +SCHR waveform might produce an additional cue to aid the discrimination tested here as long as the F0 is low enough. The smaller masking difference by +SCHR and –SCHR presented at 40 dB SPL in NH listeners suggests that the internal envelope cue may be reduced at lower levels (Leek et al. 2000).
HI listeners do not show evidence of these masking differences (Oxenham and Dau 2004; Summers and Leek 1998), suggesting that cochlear processing does not alter within-channel waveforms of the two Schroeder-phase waves for HI listeners. This would suggest that potential envelope cues would be reduced or absent for these listeners. Perhaps for the same reason, the difference in loudness between +SCHR and –SCHR is reduced in HI listeners (Mauermann and Hohmann 2007). For these listeners, only temporal fine structure cues related to the direction of the within-period frequency sweep may be available for discrimination. However, the ability of HI listeners to make full use of temporal fine structure cues has been called into question in several recent studies (Buss et al. 2004; Hopkins and Moore 2007; Hopkins et al. 2008; Lorenzi et al. 2006, 2009; Moore 2008; Moore et al. 2006).
Effects of roving level
Roving the level over a 10-dB range had little effect on performance by NH listeners but did significantly affect performance by HI listeners. This is in contrast to findings reported by Lentz and Leek (2002), who measured spectral shape discrimination by NH and HI listeners under conditions of rove and no-rove presentations. Both groups of listeners in that study demonstrated deficits in performance when the level was roved. The reduction in performance observed here for HI listeners when the level changed from one stimulus presentation to another might occur for several reasons. First, HI listeners might have performed more poorly than NH listeners on the roving level condition because the stimulus levels were inaudible for the HI listeners at the lower end of the rove range. However, the quietest stimulus was 75 dB, approximately 20 dB above thresholds for the HI listeners. Further, sounds were presented near this lower limit only on a fraction of trials, and there was no indication that HI listeners chose the louder presentation on more than half the trials. HI listeners consistently showed poor performance throughout testing on roved conditions, not just on lower-level trials.
A tempting, but ineffective, strategy for listeners would be to always choose the louder (or softer) stimulus. Such a strategy would reduce the percentage of correct responses. However, trial-by-trial analysis indicated that neither group relied on level differences within a trial to make their responses. It is possible that HI listeners did use potential loudness differences in the non-roved conditions, in which levels were fixed. This possibility might be entertained based on the significantly poorer performance of HI listeners when loudness was eliminated or reduced as a cue. That is, if there were loudness differences between the +SCHR and −SCHR, even though the intensities were at equal SPLs, HI listeners may have used that as a cue during the non-roved conditions but would have been unable to use that in the roved conditions. Two additional assumptions would have to hold to make this a plausible explanation. First, loudness differences would have to be a useful cue for HI listeners, but not used by NH listeners, as there were no differences between roved and non-roved conditions for NH listeners. One might make this argument, however, if NH listeners had access to other cues that were not available to HI listeners; therefore, NH listeners did not use a loudness cue. Second, while there is some evidence of loudness differences between the Schroeder-phase complexes in NH listeners, HI listeners experience those differences at a reduced level, if at all (Kubli et al. 2005; Mauermann and Hohmann 2007). Thus, assuming the use of a loudness cue by HI listeners is somewhat problematic. Both of these factors suggest that the reduction of performance in the roved condition is not a reflection of a difference in availability of loudness cues between the fixed and roving conditions.
Another possible explanation for the reduction in performance by HI listeners in the roved conditions might be an effect of stimulus uncertainty. Randomly roving the level increases the complexity of the task, reduces the reliability of possible loudness cues, and introduces a degree of stimulus uncertainty on each trial. Watson and his colleagues have shown that discrimination along a number of auditory dimensions is poorer when the stimuli change from presentation to presentation, even if the changes occur on dimensions irrelevant to the discrimination (e.g., Watson et al. 1976). If HI listeners were operating “close to the edge” because the sensation levels of the stimuli were low, the experimental uncertainty produced by roving level may have disrupted performance in a manner not experienced by NH listeners, who were immune to such interference when listening at a high SL. This mostly cognitive effect would be closely related to informational masking (see, e.g., Kidd et al. 2002, 2008) or perhaps to other attentional factors that might have been less than optimal due to a combination of causes including low SL, stimulus uncertainty, and the consequence of long-term hearing loss or aging of the auditory system. Indeed, several studies have suggested that HI listeners are less able than NH listeners to use available perceptual cues to overcome the effects of informational masking (Kidd et al. 2002; Arbogast et al. 2005).
In fact, performance on the rove condition may more closely mimic how HI listeners perceive dynamic sounds in the real world outside the laboratory. Natural sounds are often unpredictable and frequently fluctuating in loudness, spectral information, and temporal information. There is considerable anecdotal and laboratory-based evidence that HI listeners may be more affected than NH listeners by cognitive aspects of the listening experience such as expectancy and uncertainty (e.g., Beck and Clark 2009; Arbogast et al. 2005). Perhaps a basic characteristic of hearing loss is a reduced tolerance for stimulus uncertainty due to the combined interference of stressors such as listening at low SLs, distortion due to cochlear damage, and a generally poorer signal-to-noise ratio in the impaired ear.
Possible effects of age
Although there was overlap in age between the HI and NH listeners in this study, there were still considerable differences. The older age of the HI group may have contributed to the differences in performance in the roving condition. Previous work has shown that performance on temporal discrimination or speech discrimination tasks is negatively affected by high stimulus uncertainty conditions in elderly listeners with and without hearing loss (e.g., Fitzgibbons and Gordon-Salant 1995, 2001; Gordon-Salant 1987; Sommers et al. 1997; Trainor and Trehub 1989). Also, elderly listeners are thought to be more affected by loss of temporal fine structure cues, relative to younger listeners (Pichora-Fuller et al. 2007; Frisina and Frisina 1997), perhaps due to a reduction in neural synchrony. Reduced performance in older adults under demanding listening contexts might reflect age-related central processing deficits rather than hearing loss per se (Humes 2005), though one cannot rule out the possibility that central auditory processing declines result from degraded peripheral input in HI listeners. It is not possible to fully differentiate the relative effects of age and hearing loss in the present study, but this would be an interesting focus of future studies.
Summary
Detection thresholds for +SCHR and –SCHR were approximately 40 dB higher for HI listeners, but there was no difference in detectability between +SCHR and –SCHR for either listener group. Discrimination of the temporally reversed Schroeder harmonic complexes presented at high levels generally falls to chance at lower F0s for HI listeners than for NH listeners. However, at equal (low) SLs, discrimination is poorer for NH listeners than for HI listeners. Further, the range of F0s over which the discrimination can be made is smaller when the presentation level is reduced for NH listeners. Neither group of listeners consistently chose the more intense presentation when level was roved within a trial over a 10-dB range. Nevertheless, the roving levels significantly disrupted HI listeners’ performance, while NH listeners were not affected.
Acknowledgements
This work was supported by R01 DC00626 (MRL) and DC04664 (AML). The authors are grateful to Dr. Garnett McMillan for statistical assistance, to Lina Kubli for assistance with data collection, and to three anonymous reviewers, as well as the Associate Editor, for their thoughtful comments on an earlier version of this paper. This work was supported in part by the Department of Veterans Affairs. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Marjorie R. Leek current address: National Center for Rehabilitative Auditory Research, Portland VA Medical Center, 3710 SW U.S. Veteran’s Hospital Road, Portland, OR 97239, USA
Portions of this work were presented at the 2005 Midwinter Meeting of the Association for Research in Otolaryngology, New Orleans, LA.
Contributor Information
Amanda M. Lauer, Phone: +1-410-5028628, Email: alauer2@jhmi.edu
Michelle Molis, Email: Michelle.Molis@va.gov.
Marjorie R. Leek, Email: Marjorie.Leek@va.gov
References
- ANSI. Specifications for audiometers. Washington, DC, ANSI, 2004, ANSI S3.6–2004.
- Aitken M. Modeling variance heterogeneity in normal regressing using GLIM. Appl. Stat. 36:332–339, 1987. [DOI]
- Arbogast TL, Mason CR, Kidd G, Jr. The effect of spatial separation on informational masking of speech in normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 117:2169–2180, 2005. [DOI] [PubMed]
- Beck DL, Clark JL. Audition matters more as cognition declines: cognition matters more as audition declines. Audiology Today 21:48–59, 2009.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy Stat. Soc. Series B 57:289–300, 1995.
- Buss E, Hall JW, Grose JH. Temporal fine-structure cues to speech and pure tone modulation in observers with sensorineural hearing loss. Ear Hear 25:242–250, 2004. [DOI] [PubMed]
- Buus S, Florentine M. Gap detection in normal and impaired listeners: The effect of level and frequency. In: Michelson A (ed) Time resolution in the auditory system. Berlin, Springer-Verlag, pp. 159–179, 1985.
- Carlyon RP, Datta AJ. Masking period patterns of Schroeder-phase complexes: Effects of level, number of components, and phase of flanking components. J. Acoust. Soc. Am. 101:3648–3657, 1997. [DOI] [PubMed]
- deBoer E. Auditory time constants: A paradox? In: Michelsen A (ed) Time Resolution in Auditory Systems. Berlin, Springer-Verlag, 1985.
- Dooling RJ, Leek MR, Gleich O, Dent ML. Auditory temporal resolution in birds: Discrimination of harmonic complexes. J. Acoust. Soc. Am. 112:748–759, 2002. [DOI] [PubMed]
- Drennan WR, Longnion JK, Ruffin C, Rubinstein JT. Discrimination of Schroeder-phase harmonic complexes by normal-hearing and cochlear-implant listeners. J. Assoc. Res. Otolaryngol. 9:138–149, 2008. [DOI] [PMC free article] [PubMed]
- Duifhuis H. Consequences of peripheral frequency selectivity for nonsimultaneous masking. J. Acoust. Soc. Am. 54:1471–1488, 1973. [DOI] [PubMed]
- Fitzgibbons PJ, Gordon-Salant S. Temporal gap resolution in listeners with high frequency hearing loss. J. Acoust. Soc. Am. 81:133–137, 1987. [DOI] [PubMed]
- Fitzgibbons PJ, Gordon-Salant S. Age effects on duration discrimination with simple and complex stimuli. J. Acoust. Soc. Am. 98:3140–45, 1995. [DOI] [PubMed]
- Fitzgibbons PJ, Gordon-Salant S. Aging and temporal discrimination in auditory sequences. J. Acoust. Soc. Am. 109:2955–2963, 2001. [DOI] [PubMed]
- Fitzgibbons PJ, Wightman FL. Gap detection in normal and hearing-impaired listeners. J. Acoust. Soc. Am. 72:761–765, 1982. [DOI] [PubMed]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, Wiley, 2004.
- Florentine M, Buus S. Temporal gap detection in sensorineural and simulated hearing impairments. J. Speech Hear. Res. 27:449–455, 1984. [DOI] [PubMed]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 106:5–104, 1997. [DOI] [PubMed]
- Glasberg BR, Moore BCJ. Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. J. Acoust. Soc. Am. 79:1020–1033, 1986. [DOI] [PubMed]
- Glasberg BR, Moore BCJ. Derivation of auditory filter shapes from notched-noise data. Hear Res. 47:103–138, 1990. [DOI] [PubMed]
- Glasberg BR, Moore BCJ, Bacon S. Gap detection and masking in hearing-impaired and normal-hearing subjects. J. Acoust. Soc. Am. 81:1546–1556, 1987. [DOI] [PubMed]
- Gordon-Salant S. Age-related differences in speech recognition performance as a function of test format and paradigm. Ear. Hear 8:277–282, 1987. [DOI] [PubMed]
- Grant KW, Summers V, Leek MR. Modulation rate detection and discrimination by normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 104:1051–1060, 1998. [DOI] [PubMed]
- Hopkins K, Moore BCJ. Moderate cochlear hearing loss leads to a reduced ability to use temporal fine structure information. J. Acoust. Soc. Am. 122:1055–1068, 2007. [DOI] [PubMed]
- Hopkins K, Moore BCJ, Stone MA. Effects of moderate cochlear hearing loss on the ability to benefit from temporal fine structure information in speech. J. Acoust. Soc. Am. 123:1140–1153, 2008. [DOI] [PMC free article] [PubMed]
- Humes LE. Do ‘auditory processing’ tests measure auditory processing in the elderly? Ear. Hear. 26:109–119, 2005. [DOI] [PubMed]
- Irwin RJ, Purdy SC. The minimum detectable duration of auditory signals for normal and hearing-impaired listeners. J. Acoust. Soc. Am. 71:967–974, 1982. [DOI] [PubMed]
- Irwin RJ, Hinchcliff LK, Kemp S. Temporal acuity in normal and hearing-impaired listeners. Audiology 20:234–243, 1981. [DOI] [PubMed]
- Jesteadt W, Bilger RC, Green DM, Patterson JH. Temporal acuity in listeners with sensorineural hearing loss. J. Speech Hrg. Res. 19:357–370, 1976. [DOI] [PubMed]
- Kidd G, Jr, Arbogast TL, Mason CR, Walsh CM. Informational masking in listeners with sensorineural hearing loss. J. Assoc. Res. Otolaryngol. 3:107–199, 2002. [DOI] [PMC free article] [PubMed]
- Kidd G, Jr, Mason CR, Richards VM, Gallun FJ, Durlach N. Informational masking. In: Yost WA, Popper AN, Fay RR (eds) Auditory Perception of Sound Sources. New York, Springer-Verlag, 2008.
- Kohlrausch A, Sander A. Phase effects in masking related to dispersion in the inner ear II. Masking period patterns of short targets. J. Acoust. Soc. Am. 97:1817–1829, 1995. [DOI] [PubMed]
- Kubli L, Leek MR, Dreisbach LE. Acoustic reflexes to Schroeder-phase harmonic complexes in normal-hearing and hearing-impaired individuals. Hear. Res. 202:1–12, 2005. [DOI] [PubMed]
- Lauer AM, Dooling RJ, Leek MR, Lentz JJ. Phase effects in masking by harmonic complexes in three species of birds. J. Acoust. Soc. Am. 119:1251–1259, 2006. [DOI] [PMC free article] [PubMed]
- Lauer AM, Dooling RJ, Leek MR, Poling K. Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. J. Acoust. Soc. Am. 122:3615–3627, 2007. [DOI] [PubMed]
- Leek MR, Dent ML, Dooling RJ. Masking by harmonic complexes in budgerigars (Melopsittacus undulatus). J. Acoust. Soc. Am. 107:1737–1744, 2000. [DOI] [PubMed]
- Lentz JJ, Leek MR. Psychophysical estimates of cochlear phase response: Masking by harmonic complexes. J. Assoc. Res. Otolaryngol. 2:408–422, 2001. [DOI] [PMC free article] [PubMed]
- Lentz JJ, Leek MR. Decision strategies of hearing-impaired listeners in spectral shape discrimination. J. Acoust. Soc. Am. 111:1389–1398, 2002. [DOI] [PubMed]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 49:467–477, 1971. [DOI] [PubMed]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BCJ. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc. Nat. Acad. Sci. 103:18866–18869, 2006. [DOI] [PMC free article] [PubMed]
- Lorenzi C, Debruille L, Garnier S, Fleuriot P. Abnormal processing of temporal fine structure in speech for frequencies where absolute thresholds are normal (L). J. Acoust. Soc. Am. 125:27–30, 2009. [DOI] [PubMed]
- Mauermann M, Hohmann V. Differences in loudness of positive and negative Schroeder-phase tone complexes as a function of the fundamental frequency. J. Acoust. Soc. Am. 121:1028–1039, 2007. [DOI] [PubMed]
- Moore BCJ. The role of temporal fine structure processing in pitch perception, masking, and speech perception for normal-hearing and hearing-impaired people. J. Assoc. Res. Otolaryngol. 9:399–406, 2008. [DOI] [PMC free article] [PubMed]
- Moore BCJ, Sek A. Development of a fast method for determining sensitivity to temporal fine structure. Int. J. Aud. 48:161–171, 2009. [DOI] [PubMed]
- Moore BCJ, Glasberg BR, Hopkins K. Frequency discrimination of complex tones by hearing-impaired subjects: Evidence for loss of ability to use temporal fine structure. Hear. Res. 222:16–27, 2006. [DOI] [PubMed]
- Oxenham AJ, Dau T. Towards a measure of auditory-filter phase response. J. Acoust. Soc. Am. 110:3169–3178, 2001. [DOI] [PubMed]
- Oxenham AJ, Dau T. Masker phase effects in normal-hearing and hearing-impaired listeners: Evidence for peripheral compression at low signal frequencies. J. Acoust. Soc. Am. 116:2248–2257, 2004. [DOI] [PubMed]
- Pichora-Fuller MK, Schneider BA, MacDonald E, Pass HE, Brown S. Temporal jitter disrupts speech intelligibility: A simulation of auditory aging. Hear. Res. 223:114–121, 2007. [DOI] [PubMed]
- Preece JP, Wilson RH. Detection, loudness, and discrimination of five-component tonal complexes differing in crest factor. J. Acoust. Soc. Am. 84:166–171, 1988. [DOI] [PubMed]
- Recio A, Rhode WS. Basilar membrane responses to broadband stimuli. J. Acoust. Soc. Am. 108:2281–2298, 2000. [DOI] [PubMed]
- Schroeder MR. Synthesis of low-peak-factor signals and binary sequences with low autocorrelation. IEEE Trans. Inf. Theory 16:85–89, 1970. [DOI]
- Smith BK, Sieben UK, Kolrausch A, Schroeder MR. Phase effects in masking related to dispersion in the inner ear. J. Acoust. Soc. Am. 80:1631–1637, 1986. [DOI] [PubMed]
- Sommers MS, Kirk KI, Pisoni DB. Some considerations in evaluating spoken word recognition by normal-hearing, noise-masked normal-hearing, and cochlear Implant listeners. I: The effects of response format. Ear. Hear. 18:89–99, 1997. [DOI] [PMC free article] [PubMed]
- Summers V, Leek MR. Masking of tones and speech by Schroeder-phase harmonic complexes in normally-hearing and hearing-impaired listeners. Hear. Res. 118:139–150, 1998. [DOI] [PubMed]
- Summers V, de Boer E, Nuttall AL. Basilar-membrane responses to multicomponent (Schroeder-phase) signals: Understanding intensity effects. J. Acoust. Soc. Am. 114:294–306, 2003. [DOI] [PubMed]
- Trainor LJ, Trehub SE. Aging and auditory temporal sequencing: ordering the elements of repeating tone patterns. Percept. Psychophys 45:417–426, 1989. [DOI] [PubMed]
- Tyler RS, Summerfield Q, Wood EJ, Fernandes MA. Psychoacoustic and phonetic temporal processing in normal and hearing-impaired listeners. J. Acoust. Soc. Am. 72:740–752, 1982. [DOI] [PubMed]
- Uppenkamp S, Fobel S, Patterson RD. The effects of temporal asymmetry on the detection and perception of short chirps. Hear. Res. 158:71–83, 2001. [DOI] [PubMed]
- Verhoeven K, Simonsen K, McIntyre L. Implementing false discovery rate control: increasing your power. Oikos 108:643–647, 2005. [DOI]
- Watson CS, Kelly WJ, Wroton HW. Factors in the discrimination of tonal patterns. II. Selective attention and learning under various levels of stimulus uncertainty. J. Acoust. Soc. Am. 60:1176–1186, 1976. [DOI] [PubMed]