Figure 7.
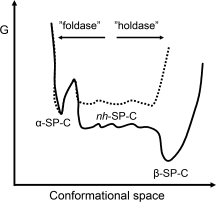
Model for the chaperone activity of CTCBrichos. The solid line represents the free energy profile for α-helical, non-helical (nh), and β-sheet SP-C, deduced from NMR hydrogen/deuterium exchange studies.24 Whether nh-SP-C has a lower energy than α-SP-C is not fully resolved.24 In the absence of CTCBrichos the poly-Val region of SP-C (the TM region of proSP-C) favors formation of β-sheet aggregates over α-helix. The proposed effect of CTCBrichos binding to the nonhelical poly-Val part (dotted line) is twofold; it favors helix formation (“foldase”) by reducing the entropic cost associated herewith, and it prevents peptide-peptide interactions required for β-sheet aggregation (“holdase”).
