Abstract
Monoclonal antibodies (mAbs) have been developed over the past years as promising anticancer therapeutics. The conjugation of tumor specific mAbs with cytotoxic molecules has been shown to improve their efficacy dramatically. These bifunctional immunotoxins, consisting of covalently linked antibodies and protein toxins, possess considerable potential in cancer therapy. Many of them are under investigation in clinical trials. As a result of general interest in new toxic components, we describe here the suitability of the bacterial protein Listeriolysin O (LLO) as cytotoxic component of an immunotoxin. Unique characteristics of LLO, such as its acidic pH optimum and the possibility to regulate the cytolytic activity by cysteine-oxidation, make LLO an interesting toxophore. Oxidized LLO shows a substantially decreased cytolytic activity when compared with the reduced protein as analyzed by hemolysis. Both oxidized and reduced LLO exhibit a cell-type-unspecific toxicity in cell culture with a significantly higher toxicity of reduced LLO. For cell-type-specific targeting of LLO to tumor cells, LLO was coupled to the dsFv fragment of the monoclonal antibody B3, which recognizes the tumor-antigen Lewis Y. The coupling of LLO to dsFv-B3 was performed via cysteine-containing polyionic fusion peptides that act as a specific heterodimerization motif. The novel immunotoxin B3-LLO could be shown to specifically eliminate antigen positive MCF7 cells with an EC50 value of 2.3 nM, whereas antigen negative cell lines were 80- to 250-fold less sensitive towards B3-LLO.
Keywords: cytotoxicity, hemolysis, disulfide bond, polyionic peptides, dimerization motif, B3, MCF7
Introduction
Antibody-based therapeutics have developed to become important constituents for treatment of human malignancies. The use of unconjugated monoclonal antibodies for cancer therapy relies on inherent biological activities such as induction of immune responses, blockage of highly expressed and activated growth-factor receptors on tumor cells, or inhibition of angiogenesis.1 Tumor-specific antibodies also provide the capability for targeting therapeutic agents to tumor cells.1,2 Protein toxins such as the bacterial diphtheria toxin, the Pseudomonas exotoxin, or the plant toxins ricin or saporin have been utilized as therapeutic agents.3 These chimaera, known as immunotoxins are described as hybrid molecules composed of an antibody or antibody fragment joined to a protein toxin.4 Antibody binding to the surface of cancer cells is followed by endocytosis of the antibody-toxin-conjugate, inducing cell death. The efficacy of the immunotoxin in eliminating tumor cells depends specifically on the number of cell surface receptors targeted by the antibody, the affinity of the antibody as well as the toxicity of the toxin. Here, we describe the suitability of the bacterial toxin Listeriolysin O (LLO) as cytotoxic part of an immunotoxin.
Listeria monocytogenes is a bacterial pathogen that grows within the cytosol of infected host cells. LLO is essential to promote the phagosomal escape of the bacterium into the cytoplasm.5–8 LLO, first characterized by Geoffroy et al.,9 belongs to a family of cholesterol-dependent pore-forming cytolysins (CDCs).10–13 CDCs insert into eukaryotic cell membranes upon cholesterol-binding, leading to oligomerization of the monomers to form pores of varying sizes.10,13,14 This ability of LLO to form pores in eukaryotic membranes is the basis of its cytolytic activity. Members of this protein family exhibit a highly conserved sequence consisting of 11 amino acids in the C-terminal domain D4 (see Fig. 1) that is involved in membrane binding16–18 and is essential for the cytolytic activity.13,16,17 A single cysteine within this undecapeptide plays an important role, as oxidation of the thiol group leads to a loss of hemolytic activity in vitro, which can be reversed completely by reduction.9,19 LLO activity is pH-dependent,9,19,20 with highest activity at acidic pH,19 corresponding to acidic cell compartments such as endosomes and lysosomes. These properties make LLO a promising cytotoxic component for an immunotoxin.
Figure 1.
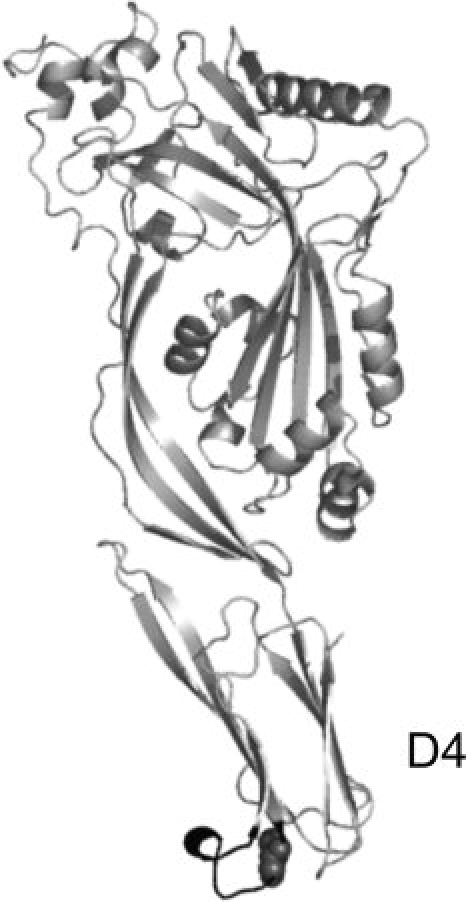
Structural image of the cholesterol-dependent cytolysin Perfringolysin O (PDB file: 1PFO;15). Perfringolysin O shares a sequence identity of 42% to LLO for the whole polypeptide chain and of 64% for the C-terminal domain D4.13 In black, the conserved sequence consisting of 11 amino acids including the singular cysteine (spheres) is shown.
We used the Fv fragment of the monoclonal antibody B3,21 stabilized by an engineered interchain disulfide bond22,23 to target LLO to tumor cells. The monoclonal antibody B3 was raised against the carbohydrate antigen Lewis Y which is highly expressed on the surface of many human tumor cells of epithelial origin like breast, lung, colon, and some epidermal cancer cells.21 Even though the antibody fragment B3 exhibits a comparably low affinity to its antigen with a dissociation constant in the micromolar range (SF and HL, unpublished data), it is highly selective21 and thus interesting as a tumor-specific targeting module of immunotoxins. Such immunotoxins have already been constructed by fusing this antibody fragment to a truncated version of the Pseudomonas exotoxin.22,24,25 The respective immunotoxins LMB-7 and LMB-9 are currently investigated in clinical trials (http://clinical trials.gov).
We utilized a highly specific heterodimerization motif for the coupling of the antibody fragment to the toxic protein LLO26 consisting of oppositely charged peptides of eight arginine and glutamic acid residues, respectively, each containing an additional cysteine residue.26 The polyionic interaction between these two peptides leads to a specific association; formation of a disulfide bond via the additional cysteine residues results in a covalently linked heterodimer. This heterodimerization motif has already been used in the design of immunotoxins27,28 and in the decoration of virus-like particles with antibody fragments successfully.29,30 The two proteins could be conjugated specifically due to the positively charged polyionic peptide fused to the disulfide stabilized antibody fragment B3 (dsFv-B3-R8C)29 and the LLO extended by the negatively charged peptide E8C (E8C-LLO). The resulting immunotoxin B3-LLO was shown to be a very efficient and cell-type-specific immunotoxin.
Results
Conjugation of the antibody fragment B3 and the toxin E8C-LLO
We used the directed and covalent association of two proteins with a specific dimerization motif consisting of the oppositely charged cysteine containing polyionic fusion peptides R8C and E8C26 to generate the immunotoxin B3-LLO.
The dsFv fragment of the monoclonal antibody B3 was C-terminal fused by the polyionic peptide R8CP,29 whereas the negatively charged peptide sequence E8C was fused to the N-terminus of LLO for coupling of LLO to the antibody fragment B3. Salt conditions below 100 mM NaCl facilitated the association of the oppositely charged fusion peptides,26 whereas copper ions catalyzed the formation of the disulfide bond between the reduced cysteines of E8C and R8CP, thus creating the covalently associated immunotoxin B3-LLO [Fig. 2(A)]. Under these conditions, the immunotoxin was formed to about 80% and could be purified from the remaining monomeric components by ion exchange chromatography.
Figure 2.
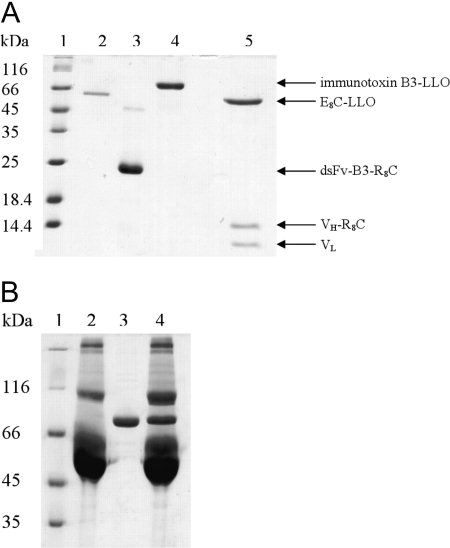
(A) Coomassie-stained SDS-PAGE analysis of the immunotoxin and single components under nonreducing conditions. Lane 1, molecular mass standard; lane 2, isolated E8C-LLO; lane 3, isolated dsFv-B3-R8C; lane 4, B3-LLO. In addition, B3-LLO was analyzed under reducing conditions, where the conjugate splits into its three parts (E8C-LLO, VH-R8CP, and VL, lane 5). (B) Analysis of the immunotoxins stability in fetal bovine serum (FBS). B3-LLO was incubated with 10% FBS for 11 h at 37°C. Subsequently, the samples were analyzed via 8% SDS-PAGE (Coomassie stained) under nonreducing conditions. Lane 1, molecular mass standard; lane 2, 10% FBS; lane 3, immunotoxin B3-LLO; lane 4, immunotoxin with 10% FBS.
For any potential applications as a therapeutic agent, B3-LLO must be stable against proteases present in serum. To analyze this, the immunotoxin was incubated with 10% fetal bovine serum (FBS, not heat inactivated) for 11 h at 37°C and afterwards analyzed via SDS-PAGE. As shown in Figure 2(B), there was no significant dissociation or degradation of B3-LLO upon incubation with FBS. Thus, the immunotoxin is sufficiently stable for analysis of its cytotoxic potential in cell culture experiments.
Functional analysis of dsFv-B3-R8C, B3-LLO and E8C-LLO
The immunotoxin B3-LLO as well as its single components were analyzed for functionality. The antibody fragment B3 and its conjugate were analyzed by antigen-dependent cell binding monitored by fluorescence microscopy and fluorescence activated cell sorting (data not shown) with fluorescein labeled proteins. The fluorescence images in Figure 3 show the binding of dsFv-B3-R8C to the Lewis Y positive MCF7 cells [Fig. 3(A)], whereas the Lewis Y negative cell line HT-29 exhibited no antibody binding [Fig. 3(B)], indicating a functional antibody fragment B3 with cell-type-specific binding activity. The polyionic fusion peptide did not influence the antigen binding.29,30 It was further investigated whether the coupling of E8C-LLO to dsFv-B3-R8C affects the Lewis Y binding of the antibody fragment. As demonstrated in Figure 3(C), the GSSG-oxidized immunotoxin B3-LLO bound to MCF7 cells but not to HT-29 cells [Fig. 3(D)], showing that the coupling of E8C-LLO to the antibody fragment B3 does not affect antigen binding. Surprisingly, the immunotoxin B3-LLO does not interact with HT-29 cells, even though the pore-forming cytolysin LLO is able to insert into eukaryotic membranes. This was not due to modification of the protein with the polyionic fusion peptide, as oxidized E8C-LLO could insert into the membranes of both MCF7 [Fig. 3(E)] and HT-29 cells [Fig. 3(F)]; coupling of the antibody fragment B3 to E8C-LLO completely abolished this membrane interaction for HT-29 cells [Fig. 3(D)]. Thus coupling of dsFv-B3-R8C to E8C-LLO generates an immunotoxin which binds specifically to the antigen Lewis Y on target cells and exhibits no unspecific cell binding mediated by E8C-LLO.
Figure 3.
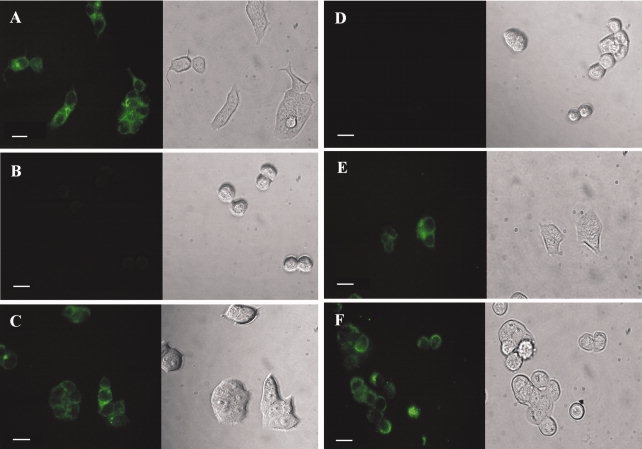
Fluorescence images of MCF7 and HT-29 cells. The cells were treated with 0.1 nM of dsFv-B3-R8C, E8C-LLO, and B3-LLO, respectively. All proteins were labeled with fluorescein. (A) MCF7 cells, positive for the antigen Lewis Y, exhibited green fluorescence after binding of the antibody fragment dsFv-B3-R8C. (B) HT-29 cells, negative for Lewis Y, did not fluoresce after incubation with dsFv-B3-R8C. (C) MCF7 cells also showed green fluorescence due to the binding of the immunotoxin B3-LLO, whereas HT-29 did not (D). The isolated cytolysin E8C-LLO interacted with both MCF7 cells (E) and HT-29 cells (F). On the left side there are fluorescence images, the right side shows the corresponding images from transmitted light. The length of the bar is equivalent to 20 μm.
Hemolysis
The interaction of LLO with cholesterol containing membranes depends initially on a highly conserved sequence consisting of 11 amino acids in its C-terminal region.16–18 This sequence contains a single cysteine whose oxidation status plays an important role for the activity of LLO as shown by hemolysis studies.9,19 Furthermore, LLO activity is pH-dependent,9 with the hemolytic activity decreased to about 30% at neutral pH in comparison to pH 5.5.19
Both E8C-LLO and B3-LLO were assayed for hemolysis. The disruption of erythrocytes was monitored by two different methods, the decrease in light scattering and the release of hemoglobin from the cells, respectively. Both methods showed identical results. Titration curves for reduced wtLLO (wtLLOred), oxidized wtLLO (wtLLOox), and the oxidized immunotoxin B3-LLO at pH 7.4 are shown in Figure 4 and EC50 values of all titrations are listed in Table I. As expected, reduced wtLLO is significantly more active than the oxidized protein. The calculated EC50 value of 38 ± 7 pM for reduced E8C-LLO at pH 7.4 is in good agreement with that for wtLLOred (21.9 ± 7 pM). Oxidized E8C-LLO exhibits an EC50 of 39.3 ± 11.7 nM, comparable with that for oxidized wtLLO (EC50 10.9 ± 1.7 nM). Thus, the polyionic fusion peptide does not change the characteristics of LLO. Under acidic conditions (pH 5.5), the hemolytic activities of reduced E8C-LLO and wtLLO are 3–7 times higher when compared with the activity at pH 7.4. Most importantly, the immunotoxin B3-LLO, with its single cysteine of E8C-LLO oxidized by GSSG, shows a very low activity with an EC50 value of 6.5 ± 1.2 nM, comparable to the activity of both oxidized wtLLO and E8C-LLO. Hemolytic activity of B3-LLO under reducing conditions could not be assayed because this leads to the dissociation of the heterobifunctional construct.
Figure 4.
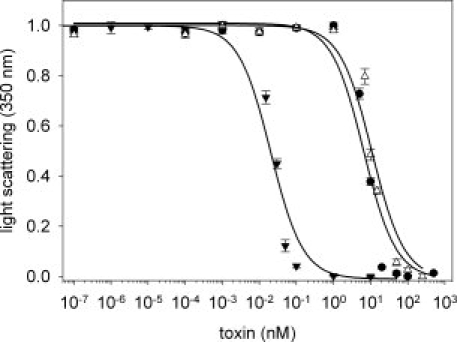
Hemolytic activity of different LLO variants. Sheep erythrocytes were treated with different concentrations of the respective LLO species and immunotoxin, respectively. After 30 min the light scattering signal at 350 nm was determined. The signal decreases due to the lysis of the erythrocytes with increasing toxin concentrations. Titrations are shown for reduced wtLLO (▾), B3-LLO (•) and oxidized wtLLO (▵) at pH 7.4. The measurements were performed in triplicate, the error bars represent the SEM of the mean value. The lines represent the fit of the experimental data to the EC50 values listed in Table I.
Table I.
Hemolytic Activity of LLO
| EC50 (pM) |
|||
|---|---|---|---|
| Toxin | Reduced (pH 7.4) | Reduced (pH 5.5) | Oxidised (pH 7.4) |
| wtLLO | 21.9 ± 7 | 7 ± 0.3 | 10.9 × 103 ± 1.7 × 103 |
| E8C-LLO | 38 ± 7 | 5.6 ± 0.7 | 39.3 × 103 ± 11.7 × 103 |
| B3-LLO | — | — | 6.5 × 103 ± 1.2 × 103 |
EC50 values of the immunotoxin and isolated toxins in reduced and oxidized form in hemolysis under physiological (PBS, pH 7.4) and acidic (citrate, pH 5.5) conditions.
These hemolysis studies show that the high cytolytic activity of LLO can be controlled by oxidation of the single cysteine of LLO in its C-terminal domain and that this reduction in activity is maintained in the immunotoxin B3-LLO.
Cytotoxicity of the immunotoxin
The conjugated immunotoxin B3-LLO was analyzed for its ability to specifically and efficiently kill tumor cells expressing the B3 antigen Lewis Y on the surface. To assay cytotoxicity, Lewis Y positive MCF7 and SKBR-3 cells and Lewis Y negative HT-29 and HeLa cells were titrated with the various protein constructs and the number of viable cells analyzed by fluorescence activated cell sorting (FACS). The immunotoxin B3-LLO was very efficient in eliminating the Lewis Y positive cell lines, with an EC50 value of 2.3 ± 0.5 nM for MCF7 [Fig. 5(A) and Table II] and of 12.7 ± 1.94 nM for SKBR-3, respectively. The slightly lower cytotoxicity of the construct for SKBR-3 when compared with MCF 7 cells is expected because SKBr-3 cells are known to possess less antigen on the cell surface.
Figure 5.
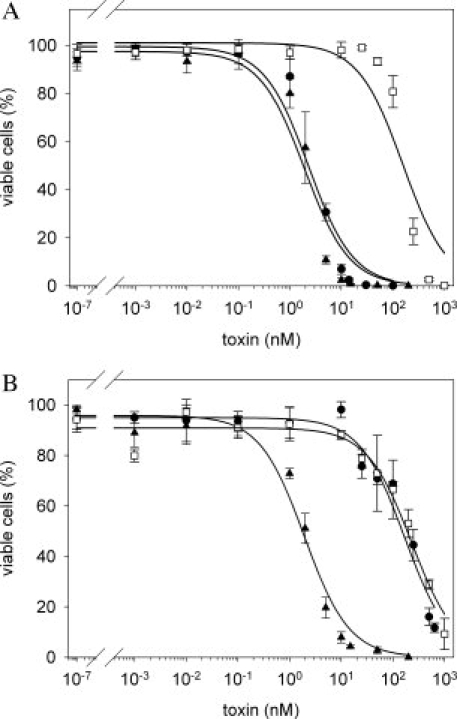
Cytotoxicity of the immunotoxin B3-LLO. MCF7 as Lewis Y positive and HT-29 as Lewis Y negative cell lines were incubated for 48 h with different concentrations of B3-LLO or the isolated toxins in reduced (E8C-LLOred) or oxidized (E8C-LLOox) form, respectively. Afterwards the cells were analyzed by FACS and the amount of viable cells was determined. All measurements were performed in triplicate, the error bars represent the SEM of the mean value. The effect of B3-LLO (•), E8C-LLOred (▴), and E8C-LLOox (□) on MCF7 cells (A) and on HT-29 cells (B) is shown. The EC50 values are given in Table II.
Table II.
Cytotoxic Activity of the Immunotoxin B3-LLO
| EC50 (nM) |
||||
|---|---|---|---|---|
| Toxin | MCF7 (Lewis Y+) | SKBR-3 (Lewis Y+) | HeLa (Lewis Y−) | HT-29 (Lewis Y−) |
| E8C-LLOred | 1.9 ± 0.4 | 2.49 ± 0.15 | 10.34 ± 2.23 | 1.9 ± 0.25 |
| B3-LLO | 2.3 ± 0.5 | 12.7 ± 1.94 | 503.78 ± 164.11 | 168.9 ± 38.9 |
| E8C-LLOox | 153.8 ± 41 | 314.8 ± 40.67 | 910.63 ± 164.07 | 231.5 ± 58.1 |
EC50 values of the immunotoxin and isolated toxins in reduced (E8C-LLOred) and oxidized form (E8C-LLOox) in cell culture experiments using different antigen positive (Lewis Y+) and antigen negative (Lewis Y−) cell lines.
In contrast, only a cell-type-unspecific cytotoxic activity (EC50 value of 168.9 ± 38.9 nM) could be determined for HT-29 cells [Fig. 5(B) and Table II] and for HeLa cells (EC50 value of 503.78 ± 164.11 nM). This low cytotoxicity is in the same range as that of the isolated oxidized E8C-LLO (E8C-LLOox) (Table II). Nevertheless, the immunotoxin B3-LLO discriminates between antigen positive and antigen negative cells, whereas E8C-LLOox is unspecific. Reduction of E8C-LLO results in a dramatic increase in cytotoxicity; E8C-LLOred exhibits the same activity as the immunotoxin B3-LLO on MCF7 and SKBR-3 cells, but is again cell-type-unspecific (Table II). It should be emphasized that the oxidized B3-LLO possesses an equivalent decreased activity as E8C-LLOox. We suggest that following antibody-mediated binding and uptake of the immunotoxin into the cell, B3-LLO is reduced intracellularly and the former inhibited activity of E8C-LLO is reversed. Following uptake of B3-LLO into antigen positive cells, the immunotoxin confers nearly the same cytotoxic activity as observed for the maximal active species E8C-LLOred.
Discussion
The treatment of cancer is characterized by a major conflict in which treatment should be as aggressive as possible to completely destroy tumor cells but with minimal side effects. To circumvent this problem, cancer therapies based on tumor-specific monoclonal antibodies (mAbs) have been developed, especially because these antibodies show only minor side effects. A couple of mAbs have already been approved as therapeutics and many others are currently in clinical trials.1,31 Despite this initial success, however, there is still an urgent need to enhance the efficacy of antibodies as anticancer therapeutics. One solution to this problem is to combine the targeting specificity of mAbs with the tumor-killing potency of cytotoxic effector molecules to produce immunoconjugates. The cytotoxic drugs used in these immunoconjugates could be chemotherapeutic agents such as, for example, antifolates,32 anthracyclines,33 radionuclides,34,35 small toxic molecules like calicheamicin,36 or protein toxins.37,38 The antibody fragment B3, used in this study, has already been evaluated in the context of immunotoxins22,24,25 and applied in clinical trials (immunotoxins LMB-7 and LMB-9; details at: http://clinicaltrials.gov). The cytotoxic potency of immunotoxins depends on several properties, such as the number of antigens on the cell-surface, the antigen-binding affinity, their internalization rate and their intracellular processing.39 To use toxins as therapeutics, they often have to be modified. For example, their binding sites for targets expressed in normal tissue have to be removed40 or they have to be deglycosylated to circumvent rapid clearance by liver cells expressing mannose receptors.41 Currently, there is no single protein toxin that has all of these ideal features. The currently used toxic parts of immunotoxins that are investigated in clinical trials are truncated variants of Pseudomonas exotoxin, gelonin, diphtheria toxin, and RNase.1 However, the search for new toxins is still in progress.
Here, we describe Listeriolysin O as a new toxic component of an immunotoxin. Because it does not require modification and shows unique characteristics, that is, the cytolytic activity that can be regulated by oxidation of the singular cysteine and the acidic pH-optimum,9,19,20 it represents an excellent candidate for novel immunotoxin generation. The cysteine in LLO serves not only as an ideal site for chemical modification and conjugation42 but also as an appropriate regulator of the hemolytic activity. Consequently, we first coupled LLO to the tumor-specific antibody fragment B3 through disulfide formation between the single cysteine of LLO and the cysteine within the R8C fusion peptide of dsFv-B3-R8C. This conjugation led to a biologically active and cell-type-specific immunotoxin; however, the yield of heterodimer formation was very low (data not shown). Coupling of the antibody fragment B3 to the protein toxin LLO via oppositely charged polyionic fusion peptides, which act as a specific heterodimerization motif26 increased the yield of conjugation product. This coupling procedure has already been exploited in the design of other immunotoxins27,28 and the modification of virus-like particles.29,30
Fusion of the polyionic fusion peptide to the N-terminus of LLO did not affect the biological activity of the protein as monitored by hemolysis. Although Geoffroy et al.9 reported that they did not observe any hemolytic activity of LLO at neutral pH it did hare a high activity under acidic conditions. This pH-dependent difference in hemolytic activity was, however, not as pronounced in a study of Giammarini et al.19,43 We could also demonstrate that LLO had hemolytic activity at neutral pH but this activity was much lower than under acidic conditions. The pH optimum of LLO activity is around pH 5.5. The oxidation of the singular cysteine of LLO leads to a dramatic loss of hemolytic activity.9,19 These characteristics were maintained in the case of the polyionic variant E8C-LLO and the immunotoxin B3-LLO. The ability to regulate its activity by cysteine-oxidation and pH makes LLO an attractive cytotoxic component.
The cell-type-specific targeting of the immunotoxin B3-LLO was mediated by the tumor-specific antibody fragment dsFv-B3-R8C. LLO exhibited only very low activity within B3-LLO because it was oxidized. Thus, the cell-type unspecific toxicity of B3-LLO was low, as demonstrated for the Lewis Y negative cell lines HT-29 and HeLa. For Lewis Y positive cells, the situation was very different due to the cell-type-specific targeting and internalization of B3-LLO. After the internalization of the immunotoxin by endocytosis into the Lewis Y positive cells, it was located in acidified endosomes/lysosomes. It is likely that the activity of E8C-LLO could be restored by intracellular reduction of B3-LLO. The reduction of disulfide bonds in endosomes and the trans-Golgi network is a well-known mechanism for activation of bacterial toxins, such as diphtheria and cholera toxin. Here, we used this concept for activation of the artificially designed immunotoxin B3-LLO. This activation results in a substantial decrease of the EC50 value for Lewis Y positive cells when compared with the control cell lines. A cell-type-specific toxicity of the immunotoxin with an EC50 value of 2.3 nM could be observed for MCF7 and of 12.7 nM for SKBR-3, respectively.
In contrast to B3-LLO, reduced E8C-LLO could eliminate all cell lines in equal measure, resulting in nearly identical EC50 values when compared with the cell-type specific activity of B3-LLO on MCF7 and SKBR-3 cells. This was surprising, because B3-LLO and E8C-LLO probably act by two different mechanisms. It is conceivable that reduced LLO binds to the cell membrane and forms pores leading to cell lysis. In contrast, the immunotoxin is internalized into cells and LLO develops its cytotoxic effect intracellularly. It is possible that after activation LLO destroys the structural integrity of the endosomes/lysosomes. Because lysosomal cathepsins have been implicated in the activation of caspases, and therefore apoptosis,44,45 the release of lysosomal proteins and enzymes into the cytoplasm could lead to cell death.45 Several studies have demonstrated that infection of cells with Listeria46 or the treatment of cells with sublytical concentrations of LLO47 leads to apoptosis. Alternatively, it is conceivable that LLO, after its release from endosomes/lysosomes, uniformly distributes throughout all cellular membranes, which could lead to cell death.
Surprisingly the EC50 values of cytotoxicity assays were approximately three orders of magnitude higher than those for hemolysis. This could be due to differences in the membrane constitution of erythrocytes and cancer cells, especially in terms of cholesterol content. Because LLO binds to cholesterol-containing membranes, a difference in the susceptibility of erythrocytes towards the cytolysin LLO in comparison with cancer cells is possible. Moreover, it could be shown that living cells are able to repair a limited number of pores,48 which also might be a reason for the differences in hemolytic and cytotoxic activity of LLO. Nevertheless, the toxicity of the immunotoxin B3-LLO with an EC50 value of 2.3 nM for MCF7 and 12.7 nM for SKBR-3 is comparable with that of other immunotoxins based on the antibody B3.24,27
Materials and Methods
Cloning, expression, and purification of toxin variant E8C-LLO
The DNA for the E8C-LLO variant was amplified from wtLLO DNA and two restriction sites (5′BspEI and 3′SalI) were introduced during PCR. The fragment was then cloned into pGEX-4T-1-vector with N-terminal polyionic extension coding for the amino acid residues E8C.27 We created the E8C-LLO variant using standard polymerase chain reaction and cloning techniques. The cloned plasmid sequence was verified by sequencing analysis. The resulting expression clone pE8C-LLO encoded a fusion protein consisting of the N-terminal glutathione-S-transferase (GST), a thrombin cleavage site between GST and the toxin, and the toxin with the N-terminal E8C extension and a C-terminal histidine6-tag. The processed N-terminal amino acid sequence of the mature toxin variant is GSPEFE8C-LLO.
The gene was expressed in the Escherichia coli strain BL21 (DE3), cultured in LB medium supplemented with 100 μg/mL ampicillin. At OD600nm = 0.8, the recombinant protein expression was induced by adding 1 mM IPTG to the medium. After induction the cultivation temperature was decreased from 37°C to 24°C. The cells were harvested 4 h after induction by centrifugation (8980g, 20 min).
For protein preparation, 25 g of wet cell paste were suspended in 50-mL buffer A (50 mM NaH2PO4/Na2HPO4 (pH 7.3), 150 mM NaCl, 5 mM DTT) with one tablet of protease inhibitor, EDTA-free (Roche). The cells were then disrupted by high pressure dispersion. The DNA in the crude extract was digested with 20 μL Benzonase (Merck) for 30 min at 20°C in the presence of 3 mM MgCl2. The extract was centrifuged for 30 min at 75,600g at 4°C in a JA30.50 rotor (Beckman Instruments). The supernatant was loaded onto a GSH-Sepharose column (GE Healthcare), equilibrated in buffer A. The column was first washed with buffer B (50 mM NaH2PO4/Na2HPO4 (pH 7.0), 300 mM NaCl, 0.1% (v/v) Tween 20, 10% (v/v) glycerol, 5 mM DTT) and then with buffer C (buffer B without Tween 20 or glycerol). The protein was eluted from the column in 50 mM NaH2PO4/Na2HPO4 (pH 8.0), 300 mM NaCl, 20 mM GSH. GST-E8C-LLO fractions were pooled and dialyzed against thrombin-cleavage buffer (20 mM Tris-HCl (pH 7.3), 200 mM NaCl, 1.5 mM KH2PO4, 80 mM MgCl2, 2.7 mM KCl). 10 U/mL thrombin (Merck) were added to the protein solution and incubated for 11 h at 20°C. Subsequently the protein was applied on a Ni-NTA HisTrap™ crude FF column (GE Healthcare), equilibrated in 50 mM NaH2PO4/Na2HPO4 (pH 7.3), 700 mM NaCl, 10 mM mercaptoethanol. GST-E8C-LLO and E8C-LLO were bound to the column, whereas GST and thrombin was found in the flow-through. After washing the column with 50 mM NaH2PO4/Na2HPO4 (pH 7.3) including 20 mM imidazole and 10 mM mercaptoethanol, the protein was eluted using a linear gradient of imidazole from 20 to 600 mM. The eluted protein was dialyzed against buffer A before the final purification step and loaded onto GSH-Sepharose column, previously equilibrated with buffer A. The flow-through contained the cleaved toxin, whereas noncleaved GST-E8C-LLO remained bound to the column. Finally, the toxin was dialyzed against storage buffer (25 mM NaH2PO4/Na2HPO4 (pH 7.3), 300 mM NaCl, 1 mM EDTA, 2 mM DTT). The concentration of the purified E8C-LLO was determined spectroscopically using an extinction coefficient of ɛ (280 nm) = 71,850 M−1cm−1.
Recombinant wtLLO was purified as described20 using a Ni-NTA HisTrap™ FF column (GE Healthcare). As an additional purification step a Poros HS column, equilibrated in 50 mM NaH2PO4/Na2HPO4 (pH 6.0) including 100 mM NaCl, 1 mM EDTA, and 5 mM DTT, was used. After washing the column with the same buffer, the bound wtLLO could be eluted using a linear gradient of 0.1–1M NaCl. The protein eluted at 300 mM salt. Finally, wtLLO was extensively dialyzed against storage buffer (50 mM NaH2PO4/Na2HPO4 (pH 6.0), 1M NaCl, 1 mM EDTA, 5 mM DTT).
Preparation of recombinant antibody fragment dsFv-B3-R8C
For targeting of tumor cells, we chose the Fv fragment of the tumor-specific antibody B3, which is directed against Lewis Y antigen.21 This Fv fragment is stabilized by an engineered interchain disulfide bond.22,23 The VH-domain with its C-terminal polyionic extension encoding the amino acid sequence R8CP and the VL-domain were expressed separately in E. coli BL21 (DE3) and isolated as inclusion bodies.49 The inclusion bodies were refolded to dsFv-B3-R8C and purified as described.27,29 The protein concentration was determined spectroscopically using an extinction coefficient of ɛ (280 nm) = 52,060 M−1 cm−1.
Conjugation of E8C-LLO and dsFv-B3-R8C
Before the conjugation of both proteins, E8C-LLO and dsFv-B3-R8C were reduced with 5 mM glutathione (GSH) for 1 h at 20°C and with 2 mM GSH for 40 min at 20°C, respectively. Excess GSH was removed by gel filtration (PD-10 column, GE Healthcare). The conjugation was performed in 25 mM NaH2PO4/Na2HPO4 (pH 8.5) including 100 mM NaCl and 1 μM CuCl2 with each protein at a concentration of 3 μM. After 20 h at 22°C, the reaction mixture was supplemented with 1 mM oxidized glutathione (GSSG) for 2 h at 22°C to oxidize the thiol group of the single cysteine of the E8C-LLO. Subsequently, the reaction mixture was shifted to a pH of 6.5 with H3PO4 and diluted 1:20 with 50 mM NaH2PO4/Na2HPO4 (pH 6.5) containing 1 mM EDTA. Subsequently, the sample was loaded onto an ion exchange column (HiTrap SP HP™, GE Healthcare), equilibrated in the same buffer. The conjugated product eluted at 30 mM NaCl in a linear gradient of NaCl from 0–1M and thus could be readily separated from the nonconjugated proteins. The protein concentration of the purified immunotoxin B3-LLO was determined spectroscopically using an extinction coefficient of ɛ (280 nm) = 123,910 M−1 cm−1.
Modification of proteins with oxidized glutathione (GSSG)
E8C-LLO and wtLLO were reduced with 5 mM DTT for 30 min at 20°C. Subsequently, excess DTT was removed by gel filtration (PD-10 column, GE Healthcare) and 50 mM GSSG was added to the protein solution. After 4 h at 22°C, the GSSG was removed by dialysis against 25 mM NaH2PO4/Na2HPO4 (pH 7.4) containing 200 mM NaCl. Quantitative oxidation of LLO with GSSG was confirmed by mass spectrometry.
Cell culture
The MCF7 (breast carcinoma) cell line was grown in RPMI 1640 Dutch Modification (PAA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco), 1% (v/v) GlutaMAX I (Gibco), 0.5% (v/v) Gentamicin (Gibco). The HT-29 (colon carcinoma) cell line was grown in Dulbecco's Modified Eagle Medium (DMEM) with 4.5 g/L glucose, with sodium pyruvate and pyridoxine (Gibco), supplemented with 10% (v/v) FBS, 1% (v/v) GlutaMAX I, and 0.5% (v/v) Gentamicin. The SKBR-3 (breast carcinoma) and HeLa (cervix carcinoma) cell lines were grown in Dulbecco's Modified Eagle Medium (DMEM) with 4.5 g/L glucose, with sodium pyruvate (PAA), supplemented with 10% (v/v) FBS, 1% (v/v) GlutaMAX I, and 0.5% (v/v) Gentamicin.
Fluorescence labeling of proteins and imaging techniques
Proteins (dsFv-B3-R8C, E8C-LLO, immunotoxin) were labeled with 5-(and 6)-carboxyfluorescein succinimidyl ester (FITC; Molecular Probes) with a spectroscopically determined degree of labeling of 2–3 molecules fluorescein per protein molecule. The labeling procedure was performed according to the manufacturer's specifications. For fluorescence images, cells of MCF7 and HT29 were trypsinized, resuspended in fresh medium, and counted. Approximately 20,000 cells of each cell line were seeded in an 8-well chamber slide (Nunc) and grown overnight. Next day, cells were washed with PBS (PAA) and incubated in incubation buffer (PBS with 3% (v/v) FBS) for 1 h at 4°C with immunotoxin-fluorescein and dsFv-B3-R8C-fluorescein, respectively, and for 30–60 min at 20°C with E8C-LLO-fluorescein. In each case, a protein concentration of 0.1 nM was used. Cells were then washed with incubation buffer and images were taken on a fluorescence microscope Axiovert 200M (Carl Zeiss), whereas FITC fluorescence was detected with the filter set 09 (excitation BP 450–490 nm, emission LP 515 nm; Carl Zeiss).
Hemolysis assay
Hemolysis describes the disruption of native erythrocytes and the release of hemoglobin from the cells. Hemolysis data were determined by measuring the differences in light-scattering of intact and lysed erythrocytes. For the hemolysis assay, sheep blood (Dade Behring) was centrifuged for 10 min at 400g (20°C). The erythrocytes were washed three times with 20 mM sodium citrate (pH 5.5) containing 150 mM NaCl for acidic conditions or PBS (PAA) at pH 7.4, respectively, and diluted to a light-scattering signal of 0.8–0.9 at 350 nm. Erythrocyte suspension (900 μL) was added to 100 μL of protein solution, which had varying protein concentrations. After an incubation time of 30 min at 37°C the light-scattering at 350 nm was determined. Alternatively, the release of hemoglobin from disrupted erythrocytes was monitored. Therefore, the cell suspension was centrifuged (5 min at 1700g, 20°C) and the absorbance of the supernatant was measured at 541 nm. The measurements were performed in 20 mM sodium citrate buffer (pH 5.5) containing 150 mM NaCl under acidic conditions and in PBS (PAA) at pH 7.4, respectively. The EC50 value of hemolysis was calculated from the titration curves according to the following equation: y = ymin + ymax/(1 + 10(log(x)−log(EC50))), where ymin = light scattering signal at the highest toxin concentration; ymax = light scattering signal at the lowest toxin concentration; x = toxin concentration.
Cytotoxicity of the immunotoxin
For the cytotoxicity assay, cells were seeded in 24-well plates with ∼25,000 cells per well and grown overnight. Next day, the cells were washed with PBS. Subsequently, medium supplemented with immunotoxin or control proteins, prediluted in PBS, was added at different concentrations. After an incubation time of 48 h (37°C, 5% CO2, humidified atmosphere), the cells were harvested by trypsinization, washed with PBS, resuspended in 10 mM HEPES/NaOH (pH 7.4) containing 140 mM NaCl and 5 mM CaCl2, and stained with 1 μg/mL propidium iodide for 15 min at 4°C. The percentage of viable cells was determined by FACS analysis. The data were fitted according to the equation y = ymin + ymax/(1 + 10(log(x)−log(IC50))), where ymin = % of surviving cells at the highest toxin concentration; ymax = % of surviving cells at the lowest toxin concentration; x = toxin concentration.
Acknowledgments
The plasmid encoding wildtype LLO (wtLLO) was a kind gift of D. Portnoy, DoMCB, Berkeley; USA. The authors thank Angelika Schierhorn, Max Planck Research Unit for Enzymology of Protein Folding, Halle; Germany for preparing the mass spectra. Milton Stubbs and Gary Sawers are acknowledged for critically reading the manuscript.
Glossary
Abbreviations:
- 8H9(dsFv)-PE38
immunotoxin composed of a disulfide stabilized Fv fragment of the monoclonal antibody 8H9 and a truncated Pseudomonas exotoxin variant
- B3-LLO
conjugated immunotoxin composed of dsFv-B3-R8C and E8C-LLO
- B3-PE38
conjugated immunotoxin composed of dsFv-B3-R8C and a truncated Pseudomonas exotoxin variant
- CDC
cholesterol-dependent cytolysin
- dsFv-B3
disulfide stabilized Fv fragment of the antibody B3
- dsFv-B3-R8C
dsFv-B3 extended at the C-terminus of the VH domain by the peptide R8CP
- DTT
Dithiothreitol
- E8C-LLO
Listeriolysin O extended at the N-terminus by the peptide E8C
- E8C-LLOox
E8C-LLO with its singular cysteine oxidized by GSSG
- E8C-LLOred
reduced E8C-LLO
- FBS
fetal bovine serum
- GST
glutathione-S-transferase
- GST-E8C-LLO
Listeriolysin O extended at the N-terminus by glutathione-S-transferase and the peptide E8C
- IPTG
Isopropyl-β-d-1-thiogalactopyranoside
- LLO
Listeriolysin O
- mAb
monoclonal antibody
- OD
optical density
- PDB
protein data bank
- VH
variable domain of the heavy chain of an antibody
- VH-R8CP
VH of the monoclonal antibody B3 extended at the C-terminus by the peptide R8CP
- VL
variable domain of the light chain of an antibody
- wt
wildtype.
References
- 1.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 2.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 3.Kreitman RJ, Pastan I. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev. 1998;31:53–88. doi: 10.1016/s0169-409x(97)00094-x. [DOI] [PubMed] [Google Scholar]
- 4.FitzGerald DJ, Kreitman R, Wilson W, Squires D, Pastan I. Recombinant immunotoxins for treating cancer. Int J Med Microbiol. 2004;293:577–582. doi: 10.1078/1438-4221-00302. [DOI] [PubMed] [Google Scholar]
- 5.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn M, Kathariou S, Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988;56:79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayley H. Toxin structure: part of a hole? Curr Biol. 1997;7:763–767. doi: 10.1016/s0960-9822(06)00399-x. [DOI] [PubMed] [Google Scholar]
- 11.Palmer M. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon. 2001;39:1681–1689. doi: 10.1016/s0041-0101(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 12.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayal S, Charbit A. Listeriolysin O: a key protein of Listeria monocytogenes with multiple functions. FEMS Microbiol Rev. 2006;30:514–529. doi: 10.1111/j.1574-6976.2006.00021.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhakdi S, Tranum-Jensen J, Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 16.Michel E, Reich KA, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Sekino-Suzuki N, Nakamura M, Mitsui KI, Ohno-Iwashita Y. Contribution of individual tryptophan residues to the structure and activity of theta-toxin (perfringolysin O), a cholesterol-binding cytolysin. Eur J Biochem. 1996;241:941–947. doi: 10.1111/j.1432-1033.1996.00941.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs T, Cima-Cabal MD, Darji A, Méndez FJ, Vázquez F, Jacobs AA, Shimada Y, Ohno-Iwashita Y, Weiss S, de los Toyos JR. The conserved undecapeptide shared by thiol-activated cytolysins is involved in membrane binding. FEBS Lett. 1999;459:463–466. doi: 10.1016/s0014-5793(99)01297-1. [DOI] [PubMed] [Google Scholar]
- 19.Giammarini C, Andreoni F, Amagliani G, Casiere A, Barocci S, Magnani M. High-level expression of the Listeria monocytogenes listeriolysin O in Escherichia coli and preliminary characterization of the purified protein. Protein Expr Purif. 2003;28:78–85. doi: 10.1016/s1046-5928(02)00682-4. [DOI] [PubMed] [Google Scholar]
- 20.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastan I, Lovelace ET, Gallo MG, Rutherford AV, Magnani JL, Willingham MC. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991;51:3781–3787. [PubMed] [Google Scholar]
- 22.Brinkmann U, Reiter Y, Jung SH, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci USA. 1993;90:7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung SH, Pastan I, Lee BK. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994;19:35–47. doi: 10.1002/prot.340190106. [DOI] [PubMed] [Google Scholar]
- 24.Reiter Y, Pai LH, Brinkmann U, Wang QC, Pastan I. Antitumor activity and pharmacokinetics in mice of a recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Cancer Res. 1994;54:2714–2718. [PubMed] [Google Scholar]
- 25.Pastan IH, Archer GE, McLendon RE, Friedman HS, Fuchs HE, Wang QC, Pai LH, Herndon J, Bigner DD. Intrathecal administration of single-chain immunotoxin, LMB-7 [B3(Fv)-PE38], produces cures of carcinomatous meningitis in a rat model. Proc Natl Acad Sci USA. 1995;28:2765–2769. doi: 10.1073/pnas.92.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter SA, Stubenrauch K, Lilie H, Rudolph R. Polyionic fusion peptides function as specific dimerization motifs. Protein Eng. 2001;14:775–783. doi: 10.1093/protein/14.10.775. [DOI] [PubMed] [Google Scholar]
- 27.Kleinschmidt M, Rudolph R, Lilie H. Design of a modular immunotoxin connected by polyionic adapter peptides. J Mol Biol. 2003;327:445–452. doi: 10.1016/s0022-2836(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 28.Kurschus FC, Kleinschmidt M, Fellows E, Dornmair K, Rudolph R, Lilie H, Jenne DE. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]
- 29.Stubenrauch K, Gleiter S, Brinkmann U, Rudolph R, Lilie H. Conjugation of an antibody Fv fragment to a virus coat protecell-specific targeting of recombinant polyoma-virus-like particles. Biochem J. 2001;356:867–873. doi: 10.1042/0264-6021:3560867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May T, Gleiter S, Lilie H. Assessment of cell type specific gene transfer of polyoma virus like particles presenting a tumor specific antibody Fv fragment. J Virol Methods. 2002;105:147–157. doi: 10.1016/s0166-0934(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 31.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 32.Shen WC, Ballou B, Ryser HJ, Hakala TR. Targeting, internalization, and cytotoxicity of methotrexate-monoclonal anti-stage-specific embryonic antigen-1 antibody conjugates in cultured F-9 teratocarcinoma cells. Cancer Res. 1986;46:3912–3916. [PubMed] [Google Scholar]
- 33.Dillman RO, Johnson DE, Shawler DL, Koziol JA. Superiority of an acid-labile daunorubicin-monoclonal antibody immunoconjugate compared to free drug. Cancer Res. 1988;48:6097–6102. [PubMed] [Google Scholar]
- 34.Press OW, Eary JF, Appelbaum FR, Martin PJ, Nelp WB, Glenn S, Fisher DR, Porter B, Matthews DC, Gooley T, Bernstein ID. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet. 1995;346:336–340. doi: 10.1016/s0140-6736(95)92225-3. [DOI] [PubMed] [Google Scholar]
- 35.Kaminski MS, Zasadny KR, Francis IR, Fenner MC, Ross CW, Milik AW, Estes J, Tuck M, Regan D, Fisher S, Glenn SD, Wahl RL. Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. J Clin Oncol. 1996;14:1974–1981. doi: 10.1200/JCO.1996.14.7.1974. [DOI] [PubMed] [Google Scholar]
- 36.Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 37.Brinkmann U, Keppler-Hafkemeyer A, Hafkemeyer P. Recombinant immunotoxins for cancer therapy. Expert Opin Biol Ther. 2001;1:693–702. doi: 10.1517/14712598.1.4.693. [DOI] [PubMed] [Google Scholar]
- 38.Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J. 2006;8:532–551. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hexham JM, Dudas D, Hugo R, Thompson J, King V, Dowling C, Neville DM, Jr, Digan ME, Lake P. Influence of relative binding affinity on efficacy in a panel of anti-CD3 scFv immunotoxins. Mol Immunol. 2001;38:397–408. doi: 10.1016/s0161-5890(01)00070-0. [DOI] [PubMed] [Google Scholar]
- 40.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–334. [PubMed] [Google Scholar]
- 41.Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Blakey DC, Newell DR. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res. 1988;48:6396–6403. [PubMed] [Google Scholar]
- 42.Saito G, Amidon GL, Lee KD. Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential. Gene Ther. 2003;10:72–83. doi: 10.1038/sj.gt.3301859. [DOI] [PubMed] [Google Scholar]
- 43.Giammarini C, Andreoni F, Amagliani G, Casiere A, Barocci S, Magnani M. Purification and characterization of a recombinant listeriolysin O expressed in Escherichia coli and possible diagnostic applications. J Biotechnol. 2004;109:13–20. doi: 10.1016/j.jbiotec.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Roberg K, Johansson U, Ollinger K. Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic Biol Med. 1999;27:1228–1237. doi: 10.1016/s0891-5849(99)00146-x. [DOI] [PubMed] [Google Scholar]
- 45.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 46.Guzmán CA, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis KN. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 47.Carrero JA, Calderon B, Unanue ER. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol. 2004;172:4866–4874. doi: 10.4049/jimmunol.172.8.4866. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs T, Darji A, Frahm N, Rohde M, Wehland J, Chakraborty T, Weiss S. Listeriolysin O: cholesterol inhibits cytolysis but not binding to cellular membranes. Mol Microbiol. 1998;28:1081–1089. doi: 10.1046/j.1365-2958.1998.00858.x. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph R, Boehm G, Lilie H. Folding proteins. In: Creighton TE, Jaenicke R, editors. Protein function—a practical approach. Oxford: IRL Press; 1997. pp. 57–99. [Google Scholar]


