Fig. 2.
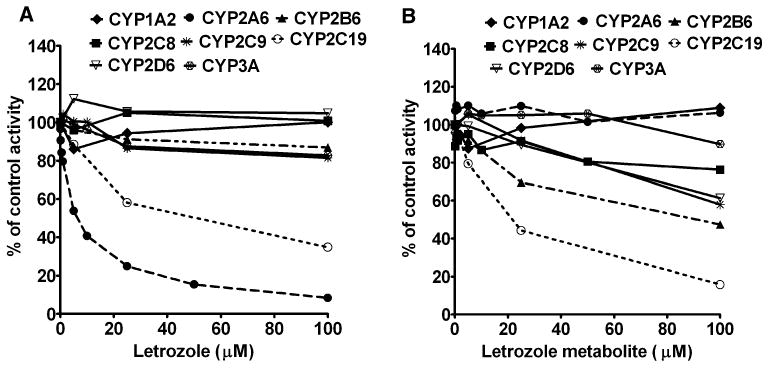
Inhibition of CYP isoforms by letrozole (a), and its metabolite 4,4′-methanol-bisbenzonitrile (b) in human liver microsomes. The activity of each enzyme was measured using substrate reaction probe selective for each isoform: phenacetin (50 μM) O-deethylation (CYP1A2); coumarin (10 μM) 7-hydroxylation (CYP2A6); efavirenz (10 μM) 8-hydroxylation (CYP2B6); amodiaquine (5 μM) desethylation (CYP2C8); tolbutamide (150 μM) 4-methyl-hydroxylation (CYP2C9); S-Mephenytoin (50 μM) 4′-hydroxylation (CYP2C19); for dextromethorphan (25 μM) O-demethylation (CYP2D6); and for midazolam (10 μM) 1′-hydroxylation (CYP3A). Each substrate probe was incubated in the absence (control) or presence of letrozole or its metabolite with HLMs and cofactors for time and protein concentrations specific for each reaction (see “Materials and methods” section). Inhibition of known CYP isoform-specific inhibitors served as positive control for inhibition. Each point represents average of duplicate incubations
