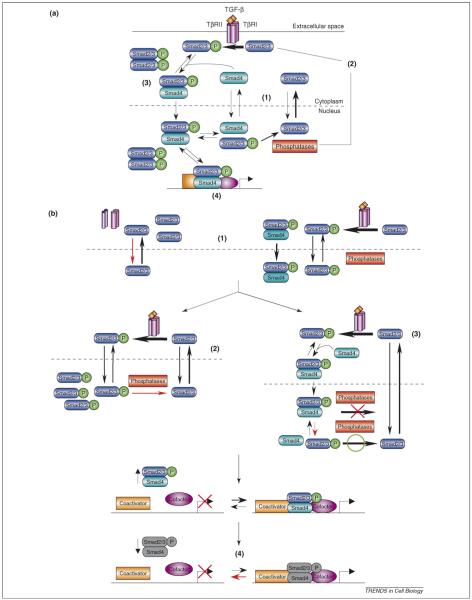
Figure 4.
Mechanisms of Smad nuclear accumulation. (a). Summary of the four postulated mechanisms for Smad nuclear accumulation in the context of canonical TGF-β/Smad signaling. (b). Smad nuclear accumulation results from several mechanisms. Red arrows indicate the rate-limiting reactions that could promote Smad nuclear accumulation. The thickness of the arrows indicates the relative reaction rates. Species that would exist at very low abundance under the assumed conditions are colored gray. In the absence of TGF-β, the Smads localize predominantly in the cytoplasm because the rate of nuclear export exceeds the rate of nuclear import [39] (1). In the presence of TGF-β signaling, enhanced nuclear import of Smad complexes compared to monomeric Smads might occur [47] (1), but most evidence to date indicates that it does not [39,40,55,56]. Furthermore, the nuclear-export machinery neither recognizes phosphorylated R-Smads strongly [57] nor Smad4 contained within Smad complexes [47,55], thereby contributing to Smad complex nuclear accumulation (1). Therefore, dissociation of Smad complexes and dephosphorylation of phospho-R-Smad are probably prerequisites for nuclear export, such that these two mechanisms represent potential causes of Smad nuclear accumulation during signaling. If dephosphorylation by the nuclear phosphatase(s) is rate-limiting, then Smad nuclear accumulation occurs if the rate of R-Smad phosphorylation is higher than that of dephosphorylation [43] (2). If dephosphorylation is not rate-limiting, then Smad oligomerization in the cytoplasm, for example with Smad4, could protect the phospho-R-Smads from the phosphatase upon nuclear translocation [43] (3). In this case, the rate at which the Smad complex dissociates in the nucleus would primarily determine the degree of Smad nuclear accumulation. A third possibility is that both mechanisms contribute substantially to Smad nuclear accumulation, which seems to be the case in vivo [47]. Finally, Smad nuclear accumulation caused by either (2) or (3) promotes the reversible binding of nuclear Smads to transcriptional cofactors, coactivators and corepressors, and DNA. Binding to such nuclear-retention factors could further sequester the phospho-R-Smads from dephosphorylation and Smad4 from the nuclear-export machinery [39,41] (4). As signaling ends, the rate of R-Smad phosphorylation decreases, which decreases the driving force for Smad complex formation. Specifically, continual phospho-R-Smad dephosphorylation depletes the concentration of monomeric phospho-R-Smads such that reversible binding reactions are driven towards dissociation, thus reducing R-Smad oligomer abundance and the abundance of Smads bound to nuclear-retention factors. The rates at which the Smad complexes dissociate probably contribute to determining the observed rate of Smad nuclear export.