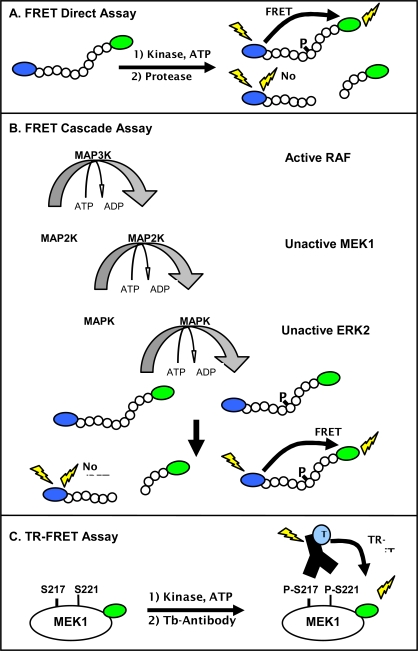
Fig. (1).
Schematic of fluorescent assay formats used to characterize kinase inhibitors within the RAF-MEK-ERK pathway. (A) The FRET direct kinase assay uses a peptide substrate terminally labeled with a coumarin-fluorescein FRET pair and measures the amount of phosphorylated product due to a decrease in sensitivity of the phosphorylated peptide to proteolysis. Proteolysis of the non-phosphorylated product decreases the FRET, while FRET is maintained in the phosphorylated peptide. (B) The FRET triple cascade kinase assay detects phosphorylation of a labeled peptide substrate, as described for a direct assay. However, the kinase reaction components vary. In the case of the triple cascade assay shown, the kinase reaction components are active MAP3K kinase (RAF), unactivated MAP2K (MEK1), unactivated MAPK (ERK2), and the FRET peptide substrate. (C) The TR-FRET format detects association between a fluorescein-labeled, phosphorylated MEK1 protein and a terbium-labeled phospho- [Ser 217/221] MEK1 antibody. RAF activity is detected by an increase in the fluorescence intensity of the fluorescein acceptor MEK1 relative to the intensity of the terbium phospho-antibody donor.