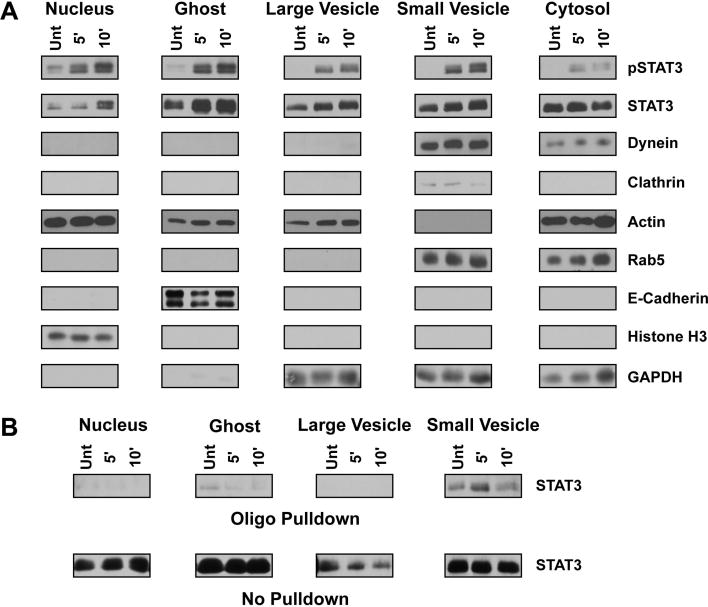
Figure 4.
Biochemical characterization and functional assay utilizing subcellular fractions. (A) Western blot analysis of subcellular fractions isolated from 3T3 MEF WT cells treated with 10 ng/ml IL-6 for the indicated period as described within the materials and methods section. STAT3, found in all subcellular fractions, is enriched within the nuclear fraction following IL-6 treatment. Activated STAT3 may also be found within the ghost, small vesicle, and large vesicle fractions. Rab5, dynein and clathrin are all found within the small vesicle fraction, indicating this fraction is enriched with early endosomes and clathrin-coated vesicles. The complete localization of e-cadherin and histone H3 to the ghost and nuclear fractions, respectively, indicates these fractions do not contaminate the vesicular fractions. The cytosolic fraction is characterized by the presence of actin, rab5, dynein, and GAPDH, suggesting the accumulation of monomeric cytoskeletal proteins and a pool of latent proteins that interact with vesicular structures. The large vesicle fraction does not contain structures associated with early endosomes and is more thoroughly described within the text and Figure 3. (B) Western blot analysis of STAT3 DNA binding capacity following 10 ng/ml IL-6 treatment of 3T3 MEF WT cells and fraction isolation. Fractions were incubated with a biotinylated oligo containing a known STAT3 binding region and then pulled down with neutravidin agarose beads as described in the materials and methods. STAT3 isolated through oligo binding is shown in the top panel labeled ‘Oligo Pulldown’ whereas the remaining STAT3 that was unable to bind the oligo is shown in the bottom panel ‘No Pulldown’. STAT3 within the small vesicle fraction has the greatest DNA binding capacity that increases upon IL-6 treatment.