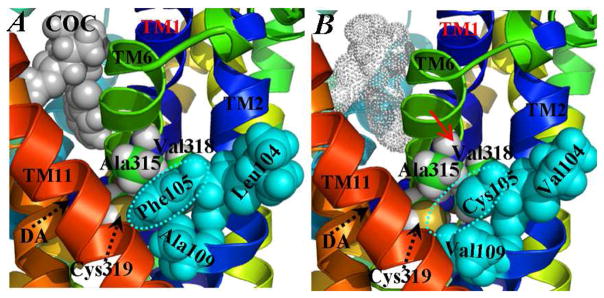
Figure 4.
Representative structural effect of Leu104Val/Phe105Cys/Ala109Val mutation on the binding of cocaine with DAT-DA. (A) Wild-type DAT-DA. The aromatic side chain of Phe105 (circled and colored in cyan) is packed with the surrounding residues as Leu104, Ala109, Ala315, Val318, and Cys319. (B) The Leu104Val/Phe105Cys/Ala109Val mutant. The same cyan colored circle shows the local hydrophobic cavity around position #105 created by the mutation. The red arrow represents the probable leaning away motion of TM6 caused by the triple mutation. The cocaine molecule is represented as dotted spheres as its binding affinity with DAT-DA is significantly reduced. In both (A) and (B), the coloring scheme and the orientation of the complex structure are the same as that in Figure 3A. Key residues are labeled and shown in space-filled spheres.