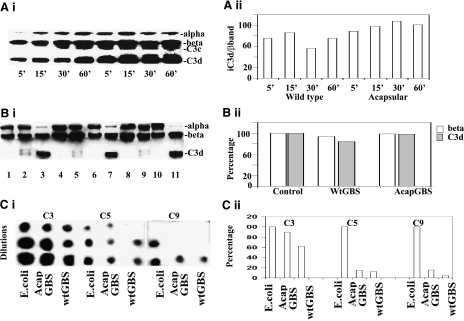
Figure 2.
Analysis of cofactor activity of FH bound to GBS. A) To analyze the cofactor activity of FH-bound GBS, wild-type and acapsular GBS were incubated in 40% NHS for various time points, boiled, and analyzed for the form by SDS-PAGE and Western blotting (i), and the relative amounts of complement C3 were assessed by densitometry and expressed as a ratio of C3d to the β band, a measure of the amount of deposition and breakdown (ii). B) i) To further test the ability of GBS-bound FH to break down C3b in solution, experiments were done with pure FH, C3b, and FI in solution. Equal colony-forming units of either wild-type or acapsular GBS were incubated with pure FH and then washed and incubated with pure C3b in the presence of FI. Bacteria were then removed by centrifugation, and 25 μl of the supernatants was subjected to Western blot analysis with an anti-C3b antibody. Lanes 1–3 show purified C3b, C3b + FI and C3b +FH + FI, respectively. Lane 3 shows complete degradation of C3b in the presence of FH and FI. Similarly, wild-type (lane 9) and acapsular GBS (lane 11) incubated with pure FH and C3b in the presence of FI show degradation of C3b similar to that of the control lane (lane 3). Other combinations were also included, in which wild-type and acapsular GBS were not incubated either with FI (lanes 8 and 10) or FH (lanes 5 and 7). ii) Amounts of C3d band and β band of lanes 3, 7, and 11 were estimated by densitometry and graphed. C) i) To demonstrate that the complement cascade was inhibited from forming because of the inactivation of C3b, the amounts of C3b, C5, and C9 deposited on the bacteria after incubation in serum were examined by dot-blot analysis using specific antibodies. E. coli K1 served as a positive control for C3b and C5 deposition, as well as C9 formation. ii) Amounts of C3, C5, and C9 in Wt GBS and acapsular GBS (AcapGBS) were estimated by densitometry and plotted in comparison to complement components formed in E. coli.