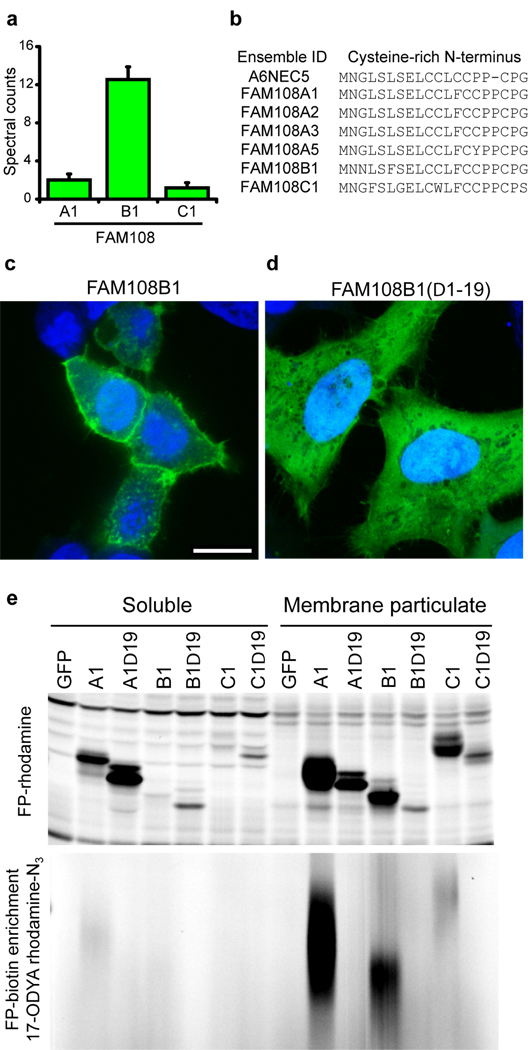
Figure 2.
Membrane tethering of the FAM108 family of serine hydrolases by palmitoylation of an N-terminal cysteine-rich motif. (a)Average spectral counts of three FAM108 proteins identified by 17-ODYA profiling of Jurkat T-cells. None of these proteins showed any spectral counts in either the palmitate or hydroxylamine controls. (b) Cysteine-rich amino acid motif conserved among the seven members of the FAM108 family of proteins. FAM108A3 and FAM108A5 (which were not identified in this study) contain an additional N-terminal sequence preceding the cysteine-rich region of 73 and 28 amino acids respectively. (c) Distribution of FAM108B1-GFP fusion protein (green) expressed in HeLa cells co-stained with DAPI (blue). Scale bar = 15 µm. (d) Distribution of FAM108B1(Δ1–19)-GFP (green) expressed in HeLa cells co-stained with DAPI (blue). Deletion of the N-terminal cysteine-rich domain prevents FAM108B1 from localizing to the plasma membrane. (e) The N-terminal cysteine-rich motif of FAM108 proteins is palmitoylated and responsible FAM108 membrane localization. FAM108 proteins are fluorophosphonate (FP)-reactive serine hydrolases, which can be efficiently labeled with activity-based probes such as FP-rhodamine or FP-biotin14. The upper panel shows FP-rhodamine-labeled soluble and membrane particulate fractions. The distribution of active FAM108 protein is altered from the particulate to soluble fractions by deletion of the N-terminal cysteine-rich motif. In the lower panel, 17-ODYA labeled transfected 293T cells were labeled with FP-biotin and enriched with streptavidin-agarose beads, reacted with rhodamine-azide, and visualized by in-gel fluorescence scanning.