Vacuolar H+-ATPases (V-ATPases) were discovered more than 30 years ago. They are related to the F-ATPases—that is, ATP synthases—but have been refined for proton pumping by the rotary ATPase activity and are responsible for the acidification of intracellular compartments. Acidification is crucial for the function of many organelles and vesicles in the cell, and yields pH gradients that are exploited by secondary transporters. Intracellular V-ATPases are therefore involved in processes such as membrane trafficking, prohormone processing, receptor-mediated endocytosis, protein degradation and the loading of synaptic vesicles. However, V-ATPases are also located in the plasma membrane, where they acidify the intercellular matrix. This activity is important in processes such as sperm cell maturation, urine acidification and bone resorption (Chatterjee et al, 1992). V-ATPases have been implicated in several human diseases, including renal tubular acidosis, osteopetrosis and cancer (Forgac, 2007). Interestingly, the ability of certain tumours to adapt to an acidic environment has been associated with malignancy, and overexpression of V-ATPases has been observed in some tumour cells (Ohta et al, 1996). The acidity of the microenvironment is an important factor in resistance to chemotherapy and might activate pH-sensitive proteases to degrade the intercellular matrix and promote metastasis. Thus, V-ATPases constitute interesting targets for anticancer drugs.
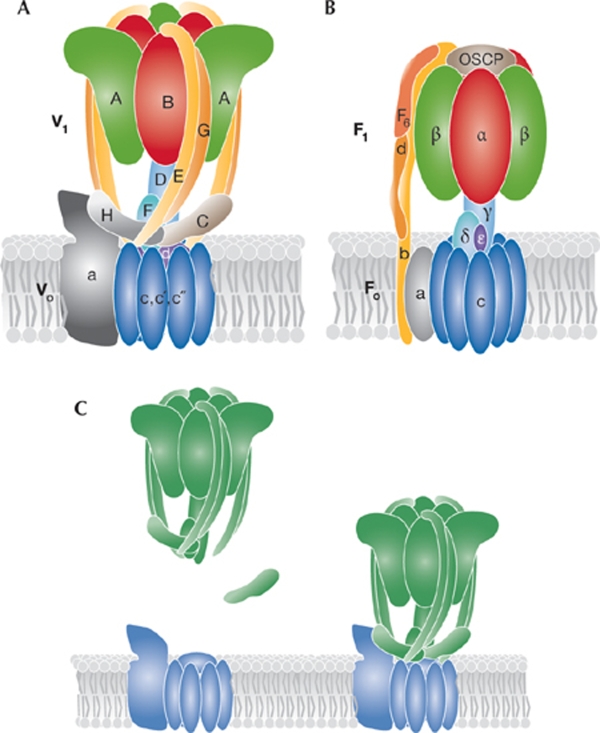
V-ATPases comprise a membrane-embedded Vo subcomplex and a cytoplasmic V1 subcomplex (Fig 1A). The Vo subcomplex consists of c, c′ and c″ subunits that form a characteristic c-ring structure, to which the a and d subunits are associated. The soluble V1 subcomplex contains a heterohexameric ring structure that comprises alternating A and B subunits. The C, D, E, F, G and H subunits associate with this A3B3 ring structure and form bridges to the Vo subcomplex. The bridging is thought to be mediated by the E and G subunits, which form three external stalk structures that interact with the a, C and H subunits (Muench et al, 2009). Furthermore, the D and F subunits—which form the central stalk structure (Makyio et al, 2005)—interact with the c-ring through the d subunit (Fig 1A).
Figure 1.
V-ATPase and F-ATPase structures and regulatory mechanism. (A) The subunit structure of the V-ATPase. The V1 subcomplex consists of the A, B, C, D, E, F, G and H subunits, whereas the Vo subcomplex consists of the a, c-ring (c, c′, c″) and d subunits. The rotor part of the proton-pumping machine is formed by the c-ring and the d, D and F subunits and is shown in different blues, whereas the ATP-hydrolysing A3B3 motor is shown in green and red. The external E/G subunit stalk structures are shown in yellow and orange and the a, C and H subunits of the membrane proximal base structure are shown in grey and dark brown. (B) The mitochondrial F-ATPase structure is shown using the same colour scheme. (C) The V-ATPases are regulated by reversible dissociation of the V1 (green) and Vo (blue) subcomplexes. When V1 leaves Vo, the C subunit dissociates from V1.
Although structural information of V-ATPases is crucial to fully understand their function and for drug design, it is still limited. The structure of the full complex has been examined by using binding studies and crosslinking, as well as electron microscopy (Gerle et al, 2006; Gregorini et al, 2007). A recent cryo-electron (cryo-EM) microscopy study of the tobacco hornworm V-ATPase at 16.5 Å resolution showed an unprecedented level of detail (Muench et al, 2009). High-resolution crystal structures of isolated subunits have recently been reported, and crystal structures of bacterial and archaeal V-ATPase homologue subunits have been determined (Saroussi & Nelson, 2009).
In this issue of EMBO reports, Numoto and colleagues take the structural investigation of V-ATPases an important step further by presenting a crystal structure of the V1-ATPase homologue from Thermus thermophilus—a well characterized enzyme that operates in the ATP synthase mode (Nakano et al, 2008)—which consists of an A3B3 hexameric ring in complex with the D and F subunits (Numoto et al, 2009). The structure has been determined at 4.5 Å resolution on the basis of experimental electron density maps; despite a low resolution, the map quality allowed model building and important structural aspects of the V-ATPases to be revealed.
In comparison to the FoF1-ATP synthases, the V-ATPases are thought to pump protons by the reverse rotation of the c-ring structure—including the D and F stalk (rotor part)—while keeping the remaining subunits static (stator part). This motion is driven by ATP hydrolysis in the A3B3 hexamer, with each AB heterodimer in a distinct nucleotide-bound or empty state at any time. Each AB state interacts specifically with the F and D subunits in the centre of the hexameric ring, and the conformational changes that result from ATP binding and hydrolysis, followed by release of ADP and Pi, drive the rotation of the F and D subunits (Fig 1A,B). The active transport of protons is thought to be mediated by rotation in the membrane of an acidic residue in the c-ring subunit that accepts a proton through a cytosolic half-channel; this proton is released when the protonated residue is exposed to a luminal (or extracellular) half-channel in the a subunit. Although a structure of a bacterial homologue of the V-ATPase c-ring has been presented (Murata et al, 2005), a high-resolution structure of the a subunit is still missing, both from V-ATPases and F-ATPases.
V-ATPase activity can be regulated by the dissociation of V1 from Vo, and other regulatory roles have been attributed to additional subunits of V-ATPases. The C subunit, for example, is important for the dissociation of the V1 subcomplex and the H subunit assists in inhibition of the dissociated V1, thereby preventing uncoupled ATPase activity (Fig 1C).
The structure of the T. thermophilus V1-ATPase solved by Numoto and colleagues shows the diameter of the A3B3 hexamer to be significantly larger than that in the F1-ATPase (Abrahams et al, 1994). This is caused by an outward protrusion from the A subunit core structure—corresponding to the β subunit of F-ATPase—which results from the insertion of a domain between the amino-terminal beta-barrel domain and the nucleotide binding domain. The empty conformation of the AB heterodimer is more open than in the corresponding F1 αβ subunits and no large-scale conformational change from the ATP-bound to ADP-bound states was observed for the A subunit. This is in contrast to the open and closed states observed for the F1-ATPase. The central stalk formed by the D and F subunits had a much straighter conformation of the D subunit than the corresponding γ subunit of F1-ATPase, which correlates well with the smaller conformational changes observed for the nucleotide-bound states of the AB subunits. Furthermore, the F subunit showed a 90° rotation compared to the orientation observed in F1. In addition, the D subunit was shown to extend through the centre of the AB heterohexamer in contrast to the internal cavity seen in a three-dimensional cryo-EM reconstruction (Muench et al, 2009). A possible explanation for this discrepancy could be the averaging of different conformational states in the cryo-EM study, although mode-dependent and species-dependent differences are also possible.
Despite constituting an important step forward, key questions remain about the fine levels of structural detail of the V1-ATPase, such as how ATP hydrolysis is coupled to a rotary proton pump function, and how the ATP synthase—and not the proton pumping—mode of operation is enforced. It is also of significant interest to establish the biological cues that select for V-type and P-type ATPase proton pumps. Proton pumping speed and a multitude of adaptive levels of functional control probably favour the multisubunit V-type ATPases for swift pH regulation, whereas high resistance against steep electrochemical gradients favours a robust P-type ATPase mechanism (Pedersen et al, 2007).
The V1-ATPase structure of the Miki laboratory constitutes a cornerstone in the V-ATPase field and brings this protein closer to the F-ATPases for comparative studies. We will also soon have access to important new structures describing V-ATPase subunits at higher resolution, such as the isolated A3B3 motor structure of the exact same T. thermophilus V1-ATPase homologue (Maher et al, 2009). However, as for all rotary ATPases, we eagerly await a high-resolution structure of a full complex.
References
- Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628 [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chakraborty M, Leit M, Neff L, Jamsa-Kellokumpu S, Fuchs R, Baron R (1992) Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton pump from other vacuolar H+ ATPases. Proc Natl Acad Sci USA 89: 6257–6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929 [DOI] [PubMed] [Google Scholar]
- Gerle C, Tani K, Yokoyama K, Tamakoshi M, Yoshida M, Fujiyoshi Y, Mitsuoka K (2006) Two-dimensional crystallization and analysis of projection images of intact Thermus thermophilus V-ATPase. J Struct Biol 153: 200–206 [DOI] [PubMed] [Google Scholar]
- Gregorini M, Wang J, Xie XS, Milligan RA, Engel A (2007) Three-dimensional reconstruction of bovine brain V-ATPase by cryo-electron microscopy and single particle analysis. J Struct Biol 158: 445–454 [DOI] [PubMed] [Google Scholar]
- Maher MJ, Akimoto S, Iwata M, Nagata K, Hori Y, Yoshida M, Yokoyama S, Iwata S, Yokoyama K (2009) Crystal structure of A3B3 complex of V-ATPase from Thermus thermophilus. EMBO J (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makyio H et al. (2005) Structure of a central stalk subunit F of prokaryotic V-type ATPase/synthase from Thermus thermophilus. EMBO J 24: 3974–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench SP, Huss M, Song CF, Phillips C, Wieczorek H, Trinick J, Harrison MA (2009) Cryo-electron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J Mol Biol 386: 989–999 [DOI] [PubMed] [Google Scholar]
- Murata T, Yamato I, Kakinuma Y, Leslie AG, Walker JE (2005) Structure of the rotor of the V-type Na+-ATPase from Enterococcus hirae. Science 308: 654–659 [DOI] [PubMed] [Google Scholar]
- Nakano M, Imamura H, Toei M, Tamakoshi M, Yoshida M, Yokoyama K (2008) ATP hydrolysis and synthesis of a rotary motor V-ATPase from Thermus thermophilus. J Biol Chem 283: 20789–20796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numoto N, Hasegawa Y, Takeda K, Miki K (2009) Inter-subunit interaction and quaternary rearrangement defined by the central stalk of prokaryotic V1-ATPase. EMBO Rep 10: 1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T et al. (1996) Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer. Br J Cancer 73: 1511–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P (2007) Crystal structure of the plasma membrane proton pump. Nature 450: 1111–1114 [DOI] [PubMed] [Google Scholar]
- Saroussi S, Nelson N (2009) The little we know on the structure and machinery of V-ATPase. J Exp Biol 212: 1604–1610 [DOI] [PubMed] [Google Scholar]