Figure 4.
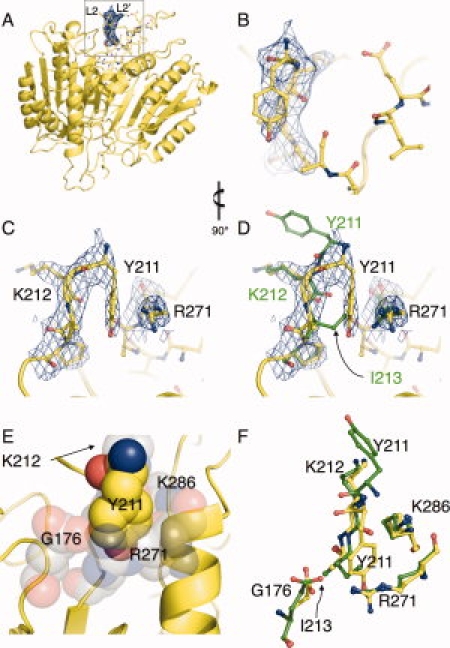
Crystallographic evidence of compensatory mechanism that facilitates active-site-binding in caspase-7 I213A. (A) Caspase-7 Y213A structure with buttress region highlighted in inset. (B) 2Fo-Fc density (blue mesh) from the initial omit map (residues 211–215 omitted from the phase calculation) and final refined model of I213A (yellow sticks) shown in same orientation as (A). (C) is as (B) but rotated 90° for clarity. The unbiased omit map clearly indicates the down position for Y211. (D) Wild-type caspase-7 (green sticks) is superimposed with the final I213A model, demonstrating that the conformation of Y211 fills a structural void when the I213 side-chain is deleted. (E) The environment (gray spheres) into which Y211 (yellow spheres) inserts is hydrophilic in nature to accommodate the hydoxyl moiety. (F) Residues forming the cavity into which Y211 inserts are in a nearly identical conformation in wild-type caspase-7 (green sticks) and I213A (yellow sticks). Figures were generated in and rendered in PyMol.7
