Figure 1.
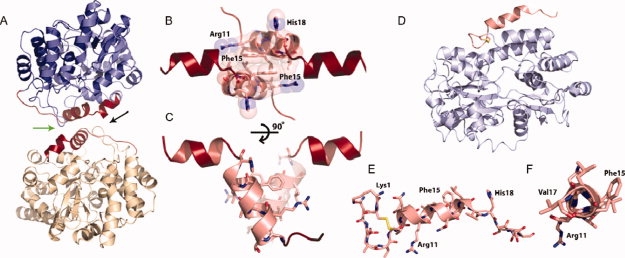
The crystal structure of the maltose binding protein (MBP)-IAPP fusions protein reveals the helical propensity of IAPP. (A) A helix-helix homodimerization interface (green arrow between IAPP molecules) is observed between fusion molecules. The two MBP molecules are shown in blue and yellow, respectively. Residues of the fusion attributable to IAPP are shown in red, each attached to MBP molecules. (B) Space filling representation showing the tight interface between parallel IAPP helices, centered at Phe 15. The view is in the direction of the black arrow in A. (C) By rotating the IAPP homodimer, it can be seen that the 8–18 helices interact at a 55° angle. (D and E) A MBP-IAPP1-22 fusion reveals the cyclic disulfide bond between Cys 2 and Cys 7 and the helical propensity within the N-terminus. MBP is shown in light blue and IAPP is shown in red. (F) View looking down the N-terminal helix of IAPP shows the 310 helix between residues Gln10 and Val17.
