Figure 4.
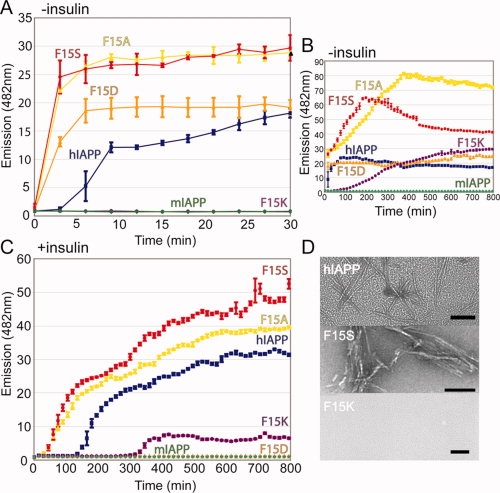
Mutations at position 15 of human IAPP affect the rate of fibril formation and the ability of insulin to inhibit this fibrillation. Residue substitutions computationally predicted to affect IAPP homodimerization and Insulin-IAPP heterodimerization were tested by a fibril formation assay. (A and B) Substitutions predicted to enhance helical association (F15A, F15S, F15D) of IAPP formed amyloid fibrils more rapidly than wild-type IAPP, as determined by Thioflavin T binding. The F15K mutation was predicted to disrupt the helical interface and a marked delay in fibril formation is observed. The 8–37 construct was used for both wild-type and Phe15 mutants. Mouse IAPP serves as a negative control for aggregation and never achieves a fluorescence value higher than baseline. (C) Including equimolar insulin in the assay delays the fibril formation of each mutant tested but has comparatively less of an inhibitory effect on the F15K substitution. (D) Representative EM images taken at 30 min of WT, F15S, and F15K to confirm the presence or absence of fibrils. Images of additional time points are included in the supporting Information. Scale bar represents 100 nm.
