Figure 1.
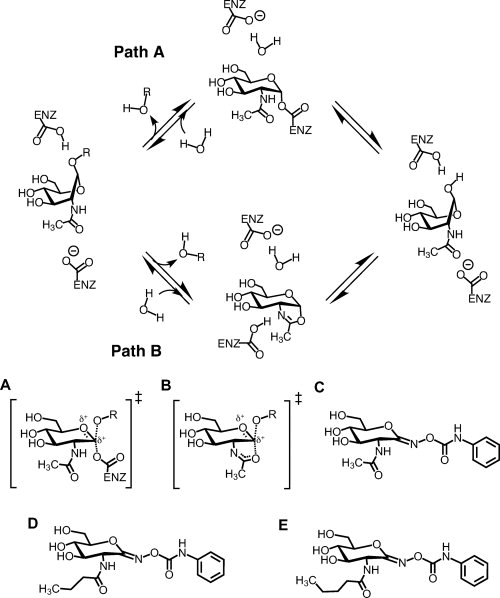
Catalytic mechanisms of family GH3 exo-N-acetyl-β-hexosaminidases (NagZ) (path A) and the family GH20 human β-hexosaminidases and GH84 O-GlcNAcase (path B), and chemical structures of PUGNAc and its 2-N-acyl derivatives. The distinguishing feature between GH3 enzymes verses GH20 and GH84 enzymes is the identity of the nucleophile. GH3 enzymes use an enzymic carboxylate, transiently forming a covalent α-glycosyl-enzyme intermediate; whereas GH20 and GH84 enzymes use a substrate assisted catalytic mechanism involving the 2-acetamido group of the substrate to form a bicyclic oxazoline or oxazolinium ion intermediate. In both cases, a molecule of water attacks the anomeric centre, breaking down the intermediate and leading to the formation of the hemiacetal product with retained stereochemistry. A: Proposed transition state for GH3 enzymes. B: Proposed transition state for GH20 and GH84 enzymes. Also shown are the chemical structures of PUGNAc (C), N-butyryl-PUGNAc (D), and N-valeryl-PUGNAc (E).
