Figure 3.
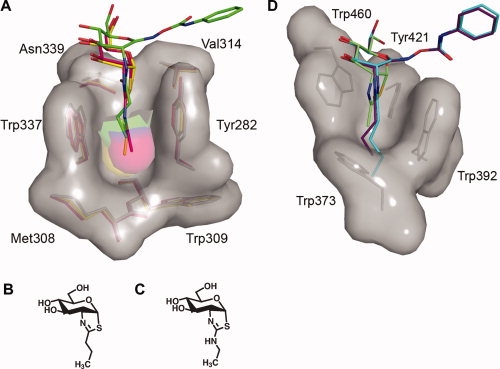
Three-dimensional structures of the 2-acetamido binding pockets of BtGH84 and human β-hexosaminidase A. A: Superposition of the BtGH84 N-butyryl-PUGNAc complex with BtGH84 bound to the thiazoline inhibitors NButGT36 and Thiamet-G37 reveals that these latter inhibitors bind deeper within the BtGH84 active site, indicating that the N-butyryl extension of N-butyryl-PUGNAc is not solely responsible for its “elevated” placement within the BtGH84 active site. N-butyryl-PUGNAc is shown with green carbon atoms, NButGT with yellow carbon atoms, and Thiamet-G with pink carbon atoms. B: Structure of Thiamet-G. C, Structure of NButGT. D: Structure of the 2-acetamido binding pocket of human β-hexosaminidase A in complex with NAG-thiazoline (PDB entry: 2GK1)25 superposed with models of N-butyryl-PUGNAc and N-valeryl-PUGNAc. The superposition clearly indicates the prohibitive steric clashes that would occur between the N-butyryl and N-valeryl groups of these inhibitors and the bottom of the shallow 2-acetamido binding pocket. Residues comprising the binding pockets of both BtGH84 and β-hexosaminidase A are shown as sticks with their corresponding solvent accessible surfaces. Superposition was carried out using CCP4.32 Figures were created with PyMOL.33
