Figure 5.
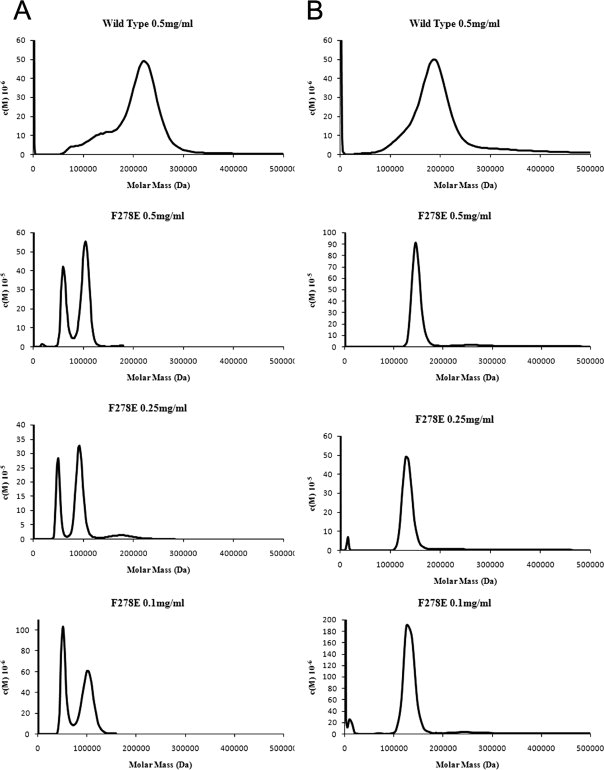
AUC analysis of the aggregation states of the human (A) and guinea pig (B) wild-type and F278E proteins; also shown is the effect of dilution on the oligomerization state of the human and guinea pig F278E enzymes. Wild-type proteins demonstrate marked heterogeneity and aggregation, whereas both F278E mutants appear far less aggregated, with the guinea pig enzyme resolving to a monodisperse tetramer, and the human protein existing as a concentration-dependent equilibrium of dimeric and tetrameric forms. Data were analyzed using SedFit27 with density, viscosity, and v-bar measurements calculated by Sednterp.
