Abstract
Molecular scale signal conversion and multiplication is of particular importance in many physical and biological applications, such as molecular switches, nano-gates, biosensors, and various neural systems. Unfortunately, little is currently known regarding the signal processing at the molecular level, partly due to the significant noises arising from the thermal fluctuations and interferences between branch signals. Here, we use molecular dynamics simulations to show that a signal at the single-electron level can be converted and multiplied into 2 or more signals by water chains confined in a narrow Y-shaped nanochannel. This remarkable transduction capability of molecular signal by Y-shaped nanochannel is found to be attributable to the surprisingly strong dipole-induced ordering of such water chains, such that the concerted water orientations in the 2 branches of the Y-shaped nanotubes can be modulated by the water orientation in the main channel. The response to the switching of the charge signal is very rapid, from a few nanoseconds to a few hundred nanoseconds. Furthermore, simulations with various water models, including TIP3P, TIP4P, and SPC/E, show that the transduction capability of the Y-shaped carbon nanotubes is very robust at room temperature, with the interference between branch signals negligible.
Keywords: confined water, molecular dynamics, molecular signal transmission, Y-shaped nanochannel, signal transduction
Understanding the signal conversion and multiplication at the molecular level is of great interest in recent years (1–4); however, due to the intrinsic complexity in these molecular systems and the significant noises arising from thermal fluctuations as well as interferences between branch signals, the molecular details remain largely unknown. Carbon nanotubes, conversely, have outstanding potential for applications such as nanoscale sensors, devices, and nanomachines (5–20). Meanwhile, water molecules confined within nanoscale channels exhibit structures and dynamics that are very different from bulk (7–12, 21–27), which might provide a medium for molecular signal transmission. Given a suitable nanochannel radius, water molecules inside the nanochannel can form a single hydrogen-bonded chain, with the “concerted” water orientations (i.e., water dipoles ordered cooperatively inside the carbon nanotube) (5–8). The characteristic time for reorientation of the dipole chain has been estimated to be in the range of 2 to 3 ns for carbon nanotubes of a length of 1.34 nm (5). Remarkably, the water molecule chains in a nanotube can remain dipole-ordered up to macroscopic lengths of approximately 0.1 mm, with durations up to approximately 0.1 s (6). Therefore, if we can “tune” the orientation of a water molecule with a charge at one end, we might be able to control the orientations of water molecules in various chains. In other words, we planned a molecular-level “signal transduction,” i.e., converting a charge signal at one end to water dipole orientation signal and then transmitting and multiplying it to many other ends.
Recently, Y-shaped nanotubes have been successfully fabricated by many different methods, including alumina templates (28), chemical vapor deposition of products generated from a pyrolysis of metallocenes (29–31), nano-welding of overlapping isolated nanotubes using high-intensity electron beams (32), and spontaneous growth of nanotube mats using Ti-doped Fe catalysts (33). Those nanotubes were found to exhibit both electrical switching and logic behavior (2, 13). Here, we show that single-file water molecules confined within a Y-shaped nano-channel can perform the signal conversion and multiplication, ignited by a single electron, due to the surprisingly strong interactions of water molecules at the Y-junction. This remarkable transduction capability is related to the fact that the concerted water orientations in the 2 branches of the Y-shaped nanotubes can be well modulated by the water orientation in the main channel. The response to the switching of the charge signal is very rapid, within a few tens of nanoseconds typically. Furthermore, our simulations with various water models, including TIP3P, TIP4P, and SPC/E, show that this transduction capability of the water-mediated signal with Y-shaped carbon nanotubes is very robust at room temperature, with the interference between branch signals negligible. To our knowledge, this is the first such observation of the remarkable water-mediated signal conversion and multiplication with a Y-shaped nanotube at a molecular level.
Results and Discussion
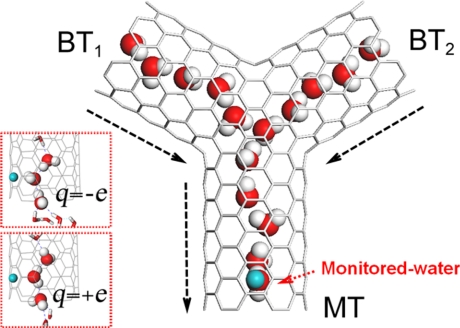
We used (6,6) uncapped armchair single-walled carbon nanotubes (SWNTs) in our Y-shaped carbon nanotube system (see Methods). Fig. 1 shows the molecular configuration of the system (hereafter referred to as Y-SWNT) together with a snapshot of the water molecules (TIP3P water model; see Methods). We have constructed the Y-SWNT by joining 3 SWNTs symmetrically, with an angle of 120° among them. An external charge q is positioned at the center of a second carbon ring of the main tube (see Fig. 1), which is used to monitor the dipole orientations of the water molecules inside the tube. All carbon atoms were fixed and an opposite charge was assigned at the edge of simulated boxes to keep the whole system electrically neutral.
Fig. 1.
A schematic snapshot of the simulation system in side view (xz plane). The Y-SWNT features a main tube (MT) and 2 branch tubes (BT1, BT2) positioned in the same plane (the xz plane). The angle between each 2 neighboring SWNTs is 120°. Small angular changes do not affect the results. The SWNTs are represented by gray lines (and the positive direction of each nanotube is shown by the dashed arrowheads). Water molecules are shown with oxygen in red and hydrogen in gray. Water molecules outside the nanotubes are omitted in the figure. The green sphere represents the imposed charge. The water molecule facing the external charge is referred to as monitored water. The lengths of MT, BT1, and BT2 are 1.44 nm, 1.21 nm, and 1.21 nm, respectively. (Insets) Close-ups for the typical configurations of the monitored water and its neighboring water molecules: q = −e (Upper) and q = +e (Lower).
We used molecular dynamics to simulate all of the water molecules in the system. The water molecules in the tubes form single hydrogen-bonded chains. Although the water chains in different tubes interact at the Y-junction, in each tube, water dipoles are often aligned with the nanotube axis, or “in concert” (i.e., water dipoles ordered cooperatively inside the channel) (5). To quantitatively describe the water dipole orientation in nanotube, an angle φi between the ith water molecule and the nanotube axis is defined as:
where p⇑i is the dipole of ith water molecule and û is the axis unit vector of the nanotube. The averaged angle φ̄ (t) is computed by the following:
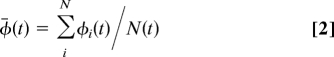 |
where the average over all of the water molecules inside a nanotube at some time t, and N(t) is the number of water molecules within this tube. The results are shown in Fig. 2A, wherein the “inward” directions of the branch tubes and the “outward” direction of the main tube are defined as positive directions (dashed arrowheads in Fig. 1). It is found that, for each nanotube, φ̄ falls into 2 ranges, 10° < φ̄ < 70° or 110° < φ̄ < 170°, for most of the time, indicating that the water molecules within each nanotube are well ordered (i.e., in concert) at any given time.
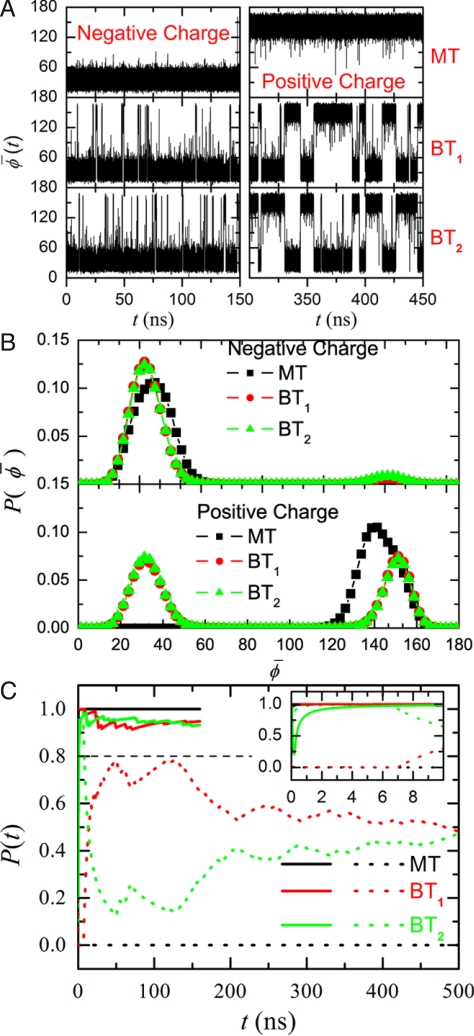
Fig. 2.
The trajectory of average dipole angle φ̄(t) of the water orientations, its distribution P(φ̄), and the probability of dipole orientation P(t) in each tube in a Y-SWNT. (A) φ̄(t) in the main tube (MT), first branch tube (BT1), and second branch tube (BT2) for a negative charge (Left) and a positive charge (Right) in the main tube. (B) Distribution of the average dipole angle φ̄(t). The statistics are obtained from the trajectory of φ̄(t) in each tube. The bin size of φ̄(t) is set at 2.5°. (C) Probability P(t) in different tubes for a negative charge (solid lines) and a positive charge (dashed lines) in the main tube. P(t) for a negative charge converges to approximately 1.0 within a few nanoseconds.
Remarkably, for q = −e, we found that φ̄(t) for all tubes falls in the range 10° < φ̄ < 70°, with few fluctuations to larger values. Fig. 2B also shows the distribution of φ̄, which clearly indicates the predominant population within 10° < φ̄ < 70° for the negative signal charge. In contrast, for q = +e, φ̄(t) for the main tube is primarily in the range 110° < φ̄ < 170°. In the case of the branch tubes, φ̄(t) fluctuates between the 2 ranges (as also shown in its distribution in Fig. 2B). Therefore, from the water orientations in each branch tube of the Y-shaped channel, we can easily distinguish the sign of the imposed charge. In other words, the charge signal at the bottom of the main tube is “converted” into water dipole signal, and then transmitted to the 2 Y-branch tops and “multiplied” (from 1 signal to 2).
To further characterize the water-mediated signal transmission, we define an integer s(t) to describe the water dipole orientations in each tube. s(t) is 1 for 10° < φ̄ < 70° and s(t) is −1 for 110° < φ̄ < 170°. We calculated the occurrence probability of s(t) = 1 with time t in each tube, denoted by P(t). It is clear that, for a sufficiently long time t→∞, P(t) approaches 1 in both branch tubes when q = −e. In contrast, P(t) approaches 0.5 in both branch tubes when q = +e, as φ̄(t) falls in the 2 different ranges with an approximately equal probability (i.e., the free energy difference estimated from histogramming analysis for the 2 states is approximately 0). Here, we set PC = 0.8 as the threshold value for determining the charge. We expect that P > PC corresponds to q = −e, and that P < PC implies q = +e. The simulation results (Fig. 2C) clearly show that, when q = −e, P > PC for any time t > 1 ns, whereas when q = +e, P < PC for any time t > 8 ns in both branch tubes. Thus, the charge signal at the main tube can be readily distinguished from the value of P(t) in each branch tube within a time interval of approximately 8 ns.
Why are there differences in the Y-branch water dipole orientations for q = −e and q = +e cases? It turns out this is related to the asymmetric charge distribution of water molecules. Careful examinations show that the charge “monitors” (or tunes) the water molecule facing this charge (referred to as “monitored water” herein). The monitored water determines the water orientations in the main tube; the uppermost water molecule in the main tube governs the dipole orientations of the lowermost water molecules in branch tubes, and consequently the water dipole orientations within both branch tubes (see Fig. S1 and Movies S1 and S2). For example, in the case of q = −e, the external charge attracts both hydrogen atoms (which have positive partial charges) of the monitored water. Then, the O atom of the monitored water strongly attracts the H atoms of its 2 nearest neighboring water molecules. Therefore, the water molecule just above the monitored water has a dipole orientation that points downward, resulting in a concerted downward orientation for all of the water molecules above the monitored water in the main tube, including the uppermost water molecule in the main tube. At the Y-junction, the O atom of the uppermost water molecule of the main tube attracts 2 H atoms, one each from the 2 lowermost water molecules of the branch tubes. Consequently, the water dipole orientation of the lowermost water molecule in the branch tubes is downward as well (i.e., +z direction), resulting in a concerted downward orientation for all of the water molecules in both branch tubes. This explains why, for the q = −e case, there are few fluctuations in water dipole orientations [i.e., s(t) stays at +1 constantly after equilibration], and the system is in a more stable state (with one O atom controlling 2 H atoms at the Y-junction). Conversely, when q = +e, the external charge attracts the O atom of the monitored water, inducing a concerted upward orientation for all of the water molecules above the monitored water in the main tube. In this case, one of the H atoms of the uppermost water molecule in the main tube will attract both O atoms in the lowermost water molecules of the branch tubes. Obviously, such a state (with one H atom controlling 2 O atoms at the Y-junction) is unfavorable and thus unstable as a result of much stronger repulsions between the 2 O atoms from the lowermost water molecules. The 2 lowermost water molecules from the branch tubes thus cannot point upward constantly. Therefore, the water dipole orientation of the lowermost water molecules, as well as all other water molecules in the branch tubes, will oscillate between upward and downward orientations (i.e., s(t) fluctuates between +1 and −1).
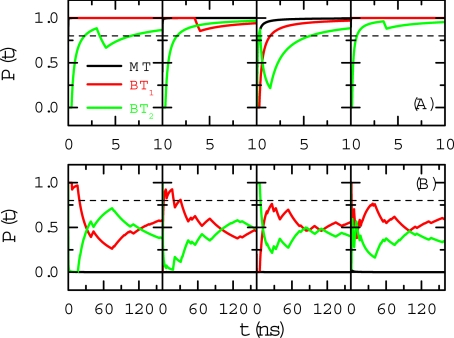
Next, we focus on the time delay of water orientation in response to a switch in the charge signal. In the aforementioned simulated systems, we recorded the water orientation states every 10 ns after the first 30 ns of simulation time. We performed new simulations from these states, but with the charge polarity switched. The averaged P(t) values for 4 such scenarios are shown in Fig. 3 A and B. The time delay associated with the branch tubes was 40 ns on average, with a maximal duration of 150 ns to respond to the −e→+e signal switch (which is slower), and approximately 4 ns only to respond to the +e→−e switch (which is much faster). The reason for this obvious disparity in the response time is the same as the different water orientation tendency discussed earlier. It is a result of the asymmetric charge distribution of water molecules. The q = −e case (in which one O atom controls 2 H atoms at the Y-junction) is much more stable than the q = +e case (in which one H atom controls 2 O atoms at the Y-junction). It is therefore more difficult and time-consuming to convert from a more stable state to a less stable state during the −e→+e transition.
Fig. 3.
Signal switching in Y-SWNT. The probability P(t) in different tubes for the imposed charge switching from positive to negative (Top) and switching from negative to positive (Bottom) for 4 typical cases with different initial states. The dipole orientations in the branch tubes fully respond in approximately 40 ns when the charge switches from negative to positive and in 4 ns in the case of positive-to-negative transitions.
Some studies (5, 34, 35) have shown that the simulation results of confined water molecules may be sensitive to the potential parameters used (e.g., reduced carbon Lennard-Jones epsilon parameters). To show the robustness of these findings, we have run extra simulations with 2 additional water models, TIP4P and SPC/E. We observed similar behavior for water dipole orientations and charge signal multiplication with 3 different water models, even though the detailed numerical values, such as the P(t) convergence time, can be slightly different. The results are shown in Fig. S2. This indicates that our results are robust with various water models used (TIP3P, TIP4P, and SPC/E). Similarly, we have also tried other angles of the Y-shaped tube (the angle among SWNTs is currently at 120°) to see if the results are sensitive to small angular changes such as those caused by fabrication errors. Obviously, an angle close to the water angle (∠HOH = 104.5°) would work best at the Y-junction. Nevertheless, we have found significant tolerance in terms of this angle, and even a T-shaped tube still shows this robust transduction capability (results shown in SI Text and Fig. S3).
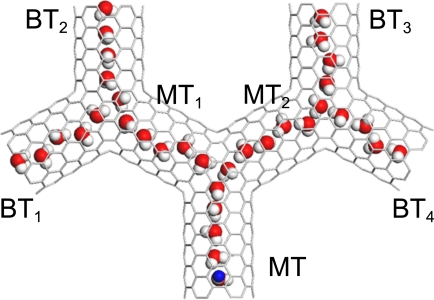
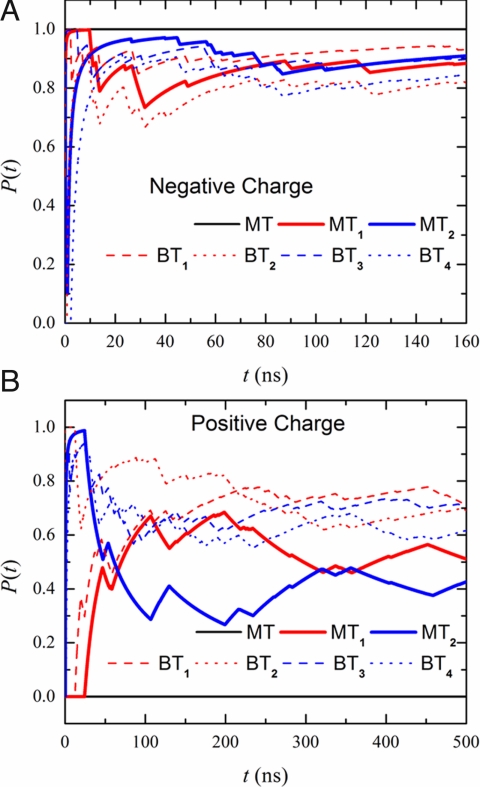
This charge-induced signal multiplication can also be applied to additional and more complicated channels. We simulated a system with 3 Y-junctions, such that each of the outlet branch tubes forms a Y-junction that connects 2 more tubes, as shown in Fig. 4. We denoted the 2 middle tubes as MT1 and MT2 and the 4 branch tubes as BT1, BT2, BT3, and BT4. The lengths of the main tube, the middle tubes, and the branch tubes were set to 1.44 nm, 1.44 nm, and 1.21 nm, respectively. Fig. 5 shows the values of P(t) for the branch tubes. It is clear that when t > 200 ns, the values of P(t) for all branch tubes are larger than PC for q = −e, and P(t) < PC when q = +e. Thus, the charge signal at the main tube is transmitted to 4 branch tubes with a temporal resolution time of approximately 200 ns.
Fig. 4.
A snapshot of a 3-Y-junction (3Y-SWNT) simulated system in a side-on orientation (xz plane). Colors match those in Fig. 1. The angle between any 2 neighboring tubes at each Y-junction is 120°. The lengths of the main tube (MT), the 2 middle tubes denoted MT1 and MT2, and the 4 branch tubes denoted BT1, BT2, BT3, and BT4 are 1.44 nm, 1.44 nm, and 1.21 nm, respectively.
Fig. 5.
Averaged values of the probability P(t) in the main tube (black line), 2 middle tubes (blue and red solid lines), and 4 branch tubes (dashed lines) for a negative (A) and a positive (B) imposed charge signal.
For practical applications, the signal of the water chain in each branch tube can be extractable from the dipole of the outermost water molecule or from the dipole moment between the ends of the water chain in each branch tube. When the orientation of the water chain is outward (inward), there is a hydrogen (oxygen) atom with a partial positive (negative) charge at the end. This partial charge can trigger (or control) a neighboring polar molecule or charge outside the channel (see the lime green water molecule in Movies S1 and S2). As each water molecule has a dipole moment, a water chain with concerted orientation has a net dipole moment, even in an environment that features thermal fluctuations. In the system shown in Fig. 1, the average dipole moment between the 2 ends of each water chain in the branch tubes is found to be 5.3 debye (D) for the case of a negative charge, averaged >150 ns. The corresponding average dipole moment is <2.7 D in the case of a positive charge. This value approaches 0 when the duration of each calculation becomes very large, i.e., the dipole moment is only 0.3 D when it is averaged across 500-ns simulations. Similarly, in this study, we have assumed a model charge of 1.0e to induce an initial charge signal. Our further simulations show that the conclusion does not change for any charge value from approximately 0.7e to 1.0e. There are several reported methods to modify the carbon nanotube surfaces to introduce such charges or charged groups at appropriate positions, such as –COOH, –OH, and –NH2 (36). Such signal charges have also been found in biological systems, such as the Arg-195 residue in the transmembrane water channel aquaporin-1 (23).
Finally, it is interesting to note that, even though we are not trying to mimic or model the biological channels with Y-shaped carbon nanotubes, these Y-shaped channels have been reported very recently in biological systems (37, 38). For example, Luna et al. described a continuous, Y-shaped, 1.8–2 nm long hydrophobic channel from 2 entrances within the lipid bilayer to heme a3-CuB center in oxidase cytochrome ba3 (37). This transmembrane channel was inferred to represent pathways for transporting O2 into and water out of the cytochrome ba3 (37). Moustafa et al. also found a Y-shaped hydrophobic channel filled with water in the crystallographic structure of L-amino acid oxidases from Calloselasma rhodostoma, whose common stem was approximately 0.9 nm (38). This channel may serve as the possible inlet for O2 (one branch of Y) and outlet for H2O2 (the other branch of Y) from the external surface of the protein to its active site (38).
Conclusion
In summary, we have shown that Y-shaped carbon nanotubes display remarkable transduction capability of water-mediated signal induced by a single electron, whereby the charge signal can be converted and multiplied into 2 or more stable water dipole orientation signals through the bifurcated branch channels. This remarkable transduction capability of Y-shaped nanotubes is observed the first time at atomic detail to our knowledge, and it is attributed to the surprisingly strong dipole-induced ordering of such water chains. The concerted water orientations in the 2 branches of the Y-shaped nanotubes can be well modulated by the water orientation in the main channel. The time delay for signal switching is in the range of several tens to hundreds of nanoseconds. Our simulations with 3 different water models (TIP3P, TIP4P, and SPC/E) show that the signal transmission capability is very robust, with the chains of water molecules in each tube remaining dipole-ordered throughout our simulation lengths (up to 500 ns). Even though these findings are from a specific size of SWNT, we expect these phenomena to be replicable for other nano-channels, provided that the water molecules inside the channels are arranged in single file. Our observations may have significance for future applications in molecular-scale electronic devices and biological systems.
Methods
Our molecular dynamics simulations were carried out in NVT ensemble at a constant temperature of 300 K using a Berendsen thermostat (39), and in constant volumes (Lx × Ly × Lz = 5.20 nm × 4.50 nm × 5.6 nm in Y-SWNT systems with 4,165 water molecules or 7.40 nm × 4.50 nm × 6.50 nm in 3Y-SWNT systems with 6,826 water molecules) using the molecular modeling package Gromacs v. 3.3.3 (40, 41). The Y-SWNT or 3Y-SWNT device was fixed at the center of simulation boxes solvated with water molecules with constant density. We adopted the particle-mesh Ewald method (42) to model long-range electrostatic interactions. We applied periodic boundary conditions in all directions. A time step of 2 fs was used, and data were collected every 0.5 ps. In all of our simulation results, the TIP3P water model was applied unless otherwise explicitly stated (2 additional water models, TIP4P and SPC/E, are also used for validation) and the carbon atoms were modeled as uncharged Lennard-Jones particles with a cross-section of σCC = 0.34 nm and σCO = 0.3275 nm and a potential well depth of εCC = 0.3612 kJ·mol−1 and εCO = 0.4802 kJ·mol−1 (5). The system was first minimized with conjugate gradient method for 10,000 steps, and then equilibrated with molecular dynamics for 5 ns at 300 K before data collection. An external signal charge of magnitude 1.0e was then introduced to the center of the second carbon ring of the main tubes (fixed during the simulations), and its opposite charge for neutralization was fixed near the edge of each box at coordinates (0.00 nm, 2.25 nm, 0.00 nm), which is far enough to have any meaningful influence on the signal charge (our numerical data also confirm this). These charges were introduced to the system after initial equilibration to observe the response time of water dipole reorientation (shown in Fig. 2) upon the charge signal.
Supplementary Material
Acknowledgments.
We thank Yongping Li, Louis Brus, Gustavo Stolovitzky, Ruwei Zhou, and Jiping Wang for helpful discussions. This work was funded by the Chinese Academy of Sciences, National Natural Science Foundation of China, Grants 10674146 and 10825520, National Basic Research Program of China Grants 2007CB936000 and 2006CB933000, the Shanghai Supercomputer Center of China, and the IBM BlueGene Science Program (to R.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902676106/DCSupplemental.
References
- 1.Kwok K, Ellenbogen J. Moletronics: Future electronics. Mater Today. 2002;5:28–37. [Google Scholar]
- 2.Xu HQ. Nanotubes: the logical choice for electronics? Nat Mater. 2005;4:649–650. doi: 10.1038/nmat1471. [DOI] [PubMed] [Google Scholar]
- 3.Koenig DR, Weig EM, Kotthaus JP. Ultrasonically driven nanomechanical single-electron shuttle. Nature Nanotechnol. 2008;3:482–485. doi: 10.1038/nnano.2008.178. [DOI] [PubMed] [Google Scholar]
- 4.Litvinchuk S, et al. Synthetic pores with reactive signal amplifiers as artificial tongues. Nat Mater. 2007;6:576–580. doi: 10.1038/nmat1933. [DOI] [PubMed] [Google Scholar]
- 5.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–190. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 6.Köfinger J, Hummer G, Dellago C. Macroscopically ordered water in nanopores. Proc Natl Acad Sci USA. 2008;105:13218–13222. doi: 10.1073/pnas.0801448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JY, et al. Electrostatic gating of a nanometer water channel. Proc Natl Acad Sci USA. 2007;104:3687–3692. doi: 10.1073/pnas.0604541104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong XJ, et al. A charge-driven molecular water pump. Nat Nanotechnol. 2007;2:709–712. doi: 10.1038/nnano.2007.320. [DOI] [PubMed] [Google Scholar]
- 9.Beckstein O, Biggin PC, Sansom MSP. A hydrophobic gating mechanism for nanopores. J Phys Chem B. 2001;105:12902–12905. [Google Scholar]
- 10.Koga K, Gao GT, Tanaka H, Zeng XC. Formation of ordered ice nanotubes inside carbon nanotubes. Nature. 2001;412:802–805. doi: 10.1038/35090532. [DOI] [PubMed] [Google Scholar]
- 11.Holt JK, et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science. 2006;312:1034–1037. doi: 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]
- 12.Majumder M, Chopra N, Andrews R, Hinds BJ. Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes. Nature. 2005;438:44. doi: 10.1038/43844a. and erratum (2005) 438:930. [DOI] [PubMed] [Google Scholar]
- 13.Bandaru PR, Daraio C, Jin S, Rao AM. Novel electrical switching behaviour and logic in carbon nanotube Y-junctions. Nat Mater. 2005;4:663–666. doi: 10.1038/nmat1450. [DOI] [PubMed] [Google Scholar]
- 14.Whitby M, Quirke N. Fluid flow in carbon nanotubes and nanopipes. Nature Nanotechnol. 2007;2:87–94. doi: 10.1038/nnano.2006.175. [DOI] [PubMed] [Google Scholar]
- 15.Bourlon B, Wong J, Miko C, Forro L, Bockrath M. A nanoscale probe for fluidic and ionic transport. Nat Nanotechnol. 2007;2:104–107. doi: 10.1038/nnano.2006.211. [DOI] [PubMed] [Google Scholar]
- 16.Bachtold A, Hadley P, Nakanishi T, Dekker C. Logic circuits with carbon nanotube transistors. Science. 2001;294:1317–1320. doi: 10.1126/science.1065824. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Sood AK, Kumar N. Carbon nanotube flow sensors. Science. 2003;299:1042–1044. doi: 10.1126/science.1079080. [DOI] [PubMed] [Google Scholar]
- 18.Fan R, Yue M, Karnik R, Majumdar A, Yang PD . Polarity switching and transient responses in single nanotube nanofluidic transistors. Phys Rev Lett. 2005;95 doi: 10.1103/PhysRevLett.95.086607. 086607. [DOI] [PubMed] [Google Scholar]
- 19.Reiter G, et al. Anomalous behavior of proton zero point motion in water confined in carbon nanotubes. Phys Rev Lett. 2006;97:247801. doi: 10.1103/PhysRevLett.97.247801. [DOI] [PubMed] [Google Scholar]
- 20.Krasheninnikov AV, Banhart F. Engineering of nanostructured carbon materials with electron or ion beams. Nat Mater. 2007;6:723–733. doi: 10.1038/nmat1996. [DOI] [PubMed] [Google Scholar]
- 21.Ball P. Water as an active constituent in cell biology. Chem Rev. 2008;108:74–108. doi: 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- 22.Zhou RH, Huang XH, Margulis CJ, Berne BJ. Hydrophobic collapse in multidomain protein folding. Science. 2004;305:1605–1609. doi: 10.1126/science.1101176. [DOI] [PubMed] [Google Scholar]
- 23.Groot BL, Grubmuller H. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- 24.Tajkhorshid E, et al. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- 25.Joseph S, Aluru NR. Why are carbon nanotubes fast transporters of water? Nano Lett. 2008;8:452–458. doi: 10.1021/nl072385q. [DOI] [PubMed] [Google Scholar]
- 26.Brewer ML, Schmitt UW, Voth GA. The formation and dynamics of proton wires in channel environments. Biophys J. 2001;80:1691–1702. doi: 10.1016/S0006-3495(01)76140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomes R, Roux B . Molecular mechanism of H+ conduction in the single-file water chain of the gramicidin channel. Biophys J. 2002;82:2304–2316. doi: 10.1016/S0006-3495(02)75576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos C, Rakitin A, Li J, Vedeneev AS, Xu JM. Electronic transport in Y-junction carbon nanotubes. Phys Rev Lett. 2000;85:3476. doi: 10.1103/PhysRevLett.85.3476. [DOI] [PubMed] [Google Scholar]
- 29.Satishkumar BC, Thomas PJ, Govindaraj A, Rao CNR. Y-junction carbon nanotubes. Appl Phys Lett. 2000;77:2530–2532. [Google Scholar]
- 30.Li WZ, Wen JG, Ren ZF. Straight carbon nanotube Y junctions. Appl Phys Lett. 2001;79:1879–1881. [Google Scholar]
- 31.Deepak FL, Govindaraj A, Rao CNR. Synthetic strategies for Y-junction carbon nanotubes. Chem Phys Lett. 2001;345:5–10. [Google Scholar]
- 32.Terrones M, et al. Molecular junctions by joining single-walled carbon nanotubes. Phys Rev Lett. 2002;89 doi: 10.1103/PhysRevLett.89.075505. 075505. [DOI] [PubMed] [Google Scholar]
- 33.Gothard N, et al. Controlled growth of Y-junction nanotubes using Ti-doped vapor catalyst. Nano Lett. 2004;4:213–217. [Google Scholar]
- 34.Krone MG, et al. Role of water in mediating the assembly of Alzheimer amyloid-beta Abeta16–22 protofilaments. J Am Chem Soc. 2008;130:11066–11072. doi: 10.1021/ja8017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhury N, Pettitt BM. On the mechanism of hydrophobic association of nanoscopic solutes. J Am Chem Soc. 2005;127:3556–3567. doi: 10.1021/ja0441817. [DOI] [PubMed] [Google Scholar]
- 36.Chopra N, Majumder M, Hinds BJ. Bifunctional carbon nanotubes by sidewall protection. J Adv Funct Mater. 2005;15:858–864. [Google Scholar]
- 37.Luna VM, Chen Y, Fee JA, Stout CD. Crystallographic studies of Xe and Kr binding within the large internal cavity of cytochrome Ba3 from Thermus thermophilus: Structural analysis and role of oxygen transport channels in the Heme-Cu oxidases. Biochemistry. 2008;47:4657–4665. doi: 10.1021/bi800045y. [DOI] [PubMed] [Google Scholar]
- 38.Moustafa IM, Foster S, Lyubimov AY, Vrielink A. Crystal structure of LAAO from Calloselasma rhodostoma with an l-phenylalanine substrate: Insights into structure and mechanism. J Mol Biol. 2006;364:991–1002. doi: 10.1016/j.jmb.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berendsen HJC, Postma JPM, Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 40.Berendsen HJC, van der Spoel D, van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput Phys Commun. 1995;91:43–56. [Google Scholar]
- 41.Lindahl E, Hess B, van der Spoel D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J Mol Model. 2001;7:306–317. [Google Scholar]
- 42.Darden T, York D, Pedersen L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.