Abstract
A circumferential marginal zone (CMZ) of retinal progenitors has been identified in most vertebrate classes, with the exception of mammals. Little is known about the formation of the CMZ during late stages of embryonic retinal histogenesis. Thus, the purpose of this study was to characterize the formation and patterning of the CMZ in the embryonic chicken retina. We identified progenitors by assaying for the expression of proliferating cell nuclear antigen (PCNA), N-cadherin and the nestin-related filament transitin, and newly generated cells by using BrdU-birthdating. We found that there is a gradual spatial restriction of progenitors into a discreet CMZ during late stages of embryonic development between E16 and hatching, at about E21. In addition, we found that retinal neurons remain immature for prolonged periods of time in far peripheral regions of the retina. Early markers of neuronal differentiation (such as HuD, calretinin and visinin) are expressed by neurons that are found directly adjacent to the CMZ. By contrast, genes that are expressed with a delay (7-10 days; PKC, calbindin, red/green opsin) after terminal mitosis in the central retina are not expressed until as much as 30 days after terminal mitosis in the far peripheral retina. We conclude that the CMZ is gradually formed during late stages of embryonic development and that the neurons that are generated by late-stage CMZ progenitors differentiate much more slowly than neurons generated during early stages of retinal development. We propose that the microenvironment within the far peripheral retina at late stages of development permits the maintenance of a zone of progenitors and slows the differentiation of neurons.
Introduction
Post-embryonic retinal growth results from on-going neurogenesis and the addition of new neurons to the peripheral edge of the retina. A zone of proliferating retinal stem cells exists at the far peripheral edge of the retinas of fish, frog and birds (reviewed by [7,22,34,40]). This zone of stem cells has been termed the ciliary or circumferential marginal zone (CMZ). In the normal mammalian retina, there is no evidence for the persistence of neural progenitors that are organized into a CMZ [4,26,33]. The CMZ is a relatively narrow band (<100 μm in diameter) of cells that line the periphery of the neural retina. It is located at the transition between the multilayered retina and the non-pigmented epithelium (NPE) of the ciliary body, a pseudostratified columnar monolayer of cells that lines the vitread border of the ciliary body. The far periphery of the retina narrows and tapers down to the CMZ, which further tapers and is continuous with the NPE of the ciliary body. In the frog eye, Perron et al [35] have demonstrated that there is a gradient of maturity that extends through the CMZ, with retinal stem cells residing in the most anterior region of the CMZ, restricted-fate progenitors residing in middle of the CMZ, and postmitotic differentiating neurons residing in the innermost region of the CMZ.
In comparison to the CMZ of frogs and fish, the avian CMZ adds relatively few retinal cells to the peripheral edge of the retina, but appears to persist into adulthood [14,26,39]. Although the CMZ of the chick can be discreetly identified with progenitor-specific markers such as transitin [12], Notch1/cHairy [7], and the combination of Pax6/Chx10 [14], progenitor-like cells may extend into the NPE of the ciliary body and retain the ability to proliferate and generate new neurons [16]. Under normal conditions, the progenitors in the CMZ are relatively quiescent, but can be stimulated to proliferate by intraocular injections of insulin, IGF-I, EGF or Shh, and by increasing rates of ocular growth via form-deprivation [10,14,16,26,32]. Conversely, the proliferation of progenitors can be suppressed by intraocular injections of glucagon or glucagon-like peptide 1 [13]. The quiescent state of the postnatal CMZ may, in part, result from glucagon-mediated inhibitory input from a unique type of retinal neuron that has been termed “bullwhip cell” [13,18]. Furthermore, under normal conditions, the progenitors in the postnatal avian CMZ do not generate photoreceptors [14], and do not produce ganglion cells unless stimulated by the combination of insulin and FGF2 [8].
Although the CMZ of the chick retina has been well described in the postnatal eye, nothing is known about the formation and patterning of the CMZ in the embryonic eye. Accordingly, the purpose of this study was to characterize the development of the CMZ in the embryonic chicken eye. We find that the CMZ is formed late during embryonic development and that there is a gradual restriction of progenitor cells to the CMZ during the last third of embryonic development. In addition, we find that the differentiation of retinal neurons near the postnatal CMZ is much slower compared to that of neurons found in central regions of the retina. We propose that the microenvironment that permits the persistence of retinal progenitors in the CMZ also slows the differentiation of neurons in the periphery of the retina.
Methods and Materials
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health and the Ohio State University. Fertilized eggs and newly hatched Leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University. Newly hatched chicks were kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am) in a stainless steel brooder at about 30°C and received water and Purinatm chick starter ad libitum.
In ovo BrdU injections
Chick embryos were staged according to guidelines established by Hamburger and Hamilton [20]. At embryonic days 8, 10, 12 or 14, we delivered 50 μg of BrdU, diluted in 20 μl of sterile saline, into the yolks of fertilized eggs. Embryos were incubated and hatched, and retinas harvested and processed for immunolabeling procedures at postnatal day 5 (P5).
Fixation, sectioning, immunohistochemistry and photography
Tissues were fixed, sectioned and immunolabeled as described elsewhere [17,19]. In short, enucleated eyes were hemisected equatorially and the gel vitreous removed from the posterior eye cup. Eye cups were fixed (4% paraformaldehyde plus 3% sucrose in 0.1 M phosphate buffer, pH 7.4, 30 min at 20°C), washed three times in PBS (phosphate-buffered saline; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4), cryoprotected in PBS plus 30% sucrose overnight, immersed in embedding medium (OCT-compound; Tissue-Tek), and mounted onto sectioning blocks. Vertical sections, nominally 12 μm thick, were cut consistently in the nasotemporal plane, and thaw-mounted onto SuperFrost Plustm slides (Fisher Scientific). Sections from control and treated eyes from the same individual were placed consecutively on each slide to ensure equal exposures to reagents. Sections were air-dried and stored at -20°C until use.
Sections were thawed, ringed with rubber cement, washed three times in PBS, covered with primary antibody solution (200 μl of antiserum diluted in PBS plus 5% normal goat serum, 0.2% Triton X-100, and 0.01% NaN3), and incubated for about 24 h at 20°C in a humidified chamber. The slides were washed three times in PBS, covered with secondary antibody solution, and incubated for at least 1 hour at 20°C in a humidified chamber. Finally, samples were washed three times in PBS, rubber cement removed from the slides, and coverglass mounted in 4:1 (v:v) glycerol to water.
Working dilutions and sources of antibodies used in this study included mouse anti-BrdU at 1:50 (G3G4; Developmental Studies Hybridoma Band (DSHB)), rat anti-BrdU at 1:100 ((OBT0030S; Serotec) mouse anti-proliferating cell nuclear antigen at 1:1000 (PCNA; Dako), mouse anti-transitin at 1:600 (7B3A5; Dr. P. Henion, Ohio State University), mouse anti-N-cadherin at 1:20 (6B3; DSHB), rabbit anti-calretinin at 1:1000 (7699/4; Swant Immunochemicals), mouse anti-visinin at 1:80 (7G4; DSHB), mouse anti-HuD at 1:200 (16A11; Invitrogen), mouse anti-synaptophysin at 1:100 (611880; BD Biosciences Pharmingen), mouse anti-PKC at 1:100 (554207; BD Biosciences Pharmingen), mouse anti-calbindin at 1:800 (300; Swant Immunochemicals), and rabbit anti-red/green opsin (AB5405; Chemicon). We evaluated the specificity of primary antibodies by comparison with published examples of results and assays for specificity [5,10,14,15,25,27,31]. Fluorescence within tissue sections was not caused by non-specific binding of the secondary antibody because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies included goat-anti-rabbit-Alexa488/568, goat-anti-mouse-Alexa488/568 and goat-anti-mouse-IgM-Alexa568 (Invitrogen) diluted to 1:1000 in PBS plus 0.2% Triton X-100. Photomicrographs were taken by using a Leica DM5000B microscope equipped with epifluorescence and a 12 megapixel Leica DC500 digital camera. Images were optimized for color, brightness and contrast, and double-labeled images overlaid by using Adobe Photoshop™6.0.
Results
Prada et al [36] utilized tritiated thymidine to determine the birthdates of cells in the chick retina and described gradients of maturation from central to peripheral and nasal to temporal retinal regions. This study however, did not characterize the production of retinal neurons in far peripheral regions of the retina and the CMZ. To determine when the cells in the periphery of the retina and the CMZ are generated, BrdU was injected into the yolk of time-staged embryos at E8, E10, E12 or E14. The embryos were allowed to hatch and the eyes were fixed at postnatal day 5. Cells with nuclei that were robustly labeled for BrdU-immunofluorescence were likely generated by progenitors that went through terminal S-phase shortly after the delivery of the BrdU. Cells with nuclei that were partially labeled with BrdU and contained only puncta of fluorescence, were likely generated subsequent to one or more divisions, and resulting dilutions of the BrdU, before terminal mitosis. Cells without nuclear BrdU-labeling were postmitotic prior to the delivery of BrdU or were labeled with BrdU and divided enough times to render the remaining BrdU undetectable.
In the far nasal regions of the postnatal retina, a single dose of BrdU applied at E8 labeled cells in the GCL within 600 μm of the CMZ, numerous cells across the ONL, and many cells in the INL (Fig. 1a). Most of the BrdU-labeled cells in the INL were at least 400 μm into the neural retina, away from the postnatal CMZ (Fig. 1a). In the far temporal regions of the postnatal retina, by comparison, a pulse of BrdU at E8 did not completely label any cells within 600 μm of the postnatal CMZ (Fig. 1b). In far nasal regions of the retina, BrdU delivered at E10 labeled the nuclei of a few cells in ONL (presumptive photoreceptors) within 300 μm of the CMZ, many cells in the INL within 800 μm of the CMZ, and a few cells in the GCL within 400 μm of the CMZ (Fig. 1c). In the far temporal regions of the postnatal retina, by comparison, BrdU delivered at E10 fully labeled the nuclei of a few cells in ONL that were at least 400 μm away from the CMZ (Fig. 1d). In addition, we observed some scattered cells that appeared to have received a full dose of BrdU in the inner and outer INL; likely amacrine and horizontal cells, respectively (Fig. 1d).
Figure 1.
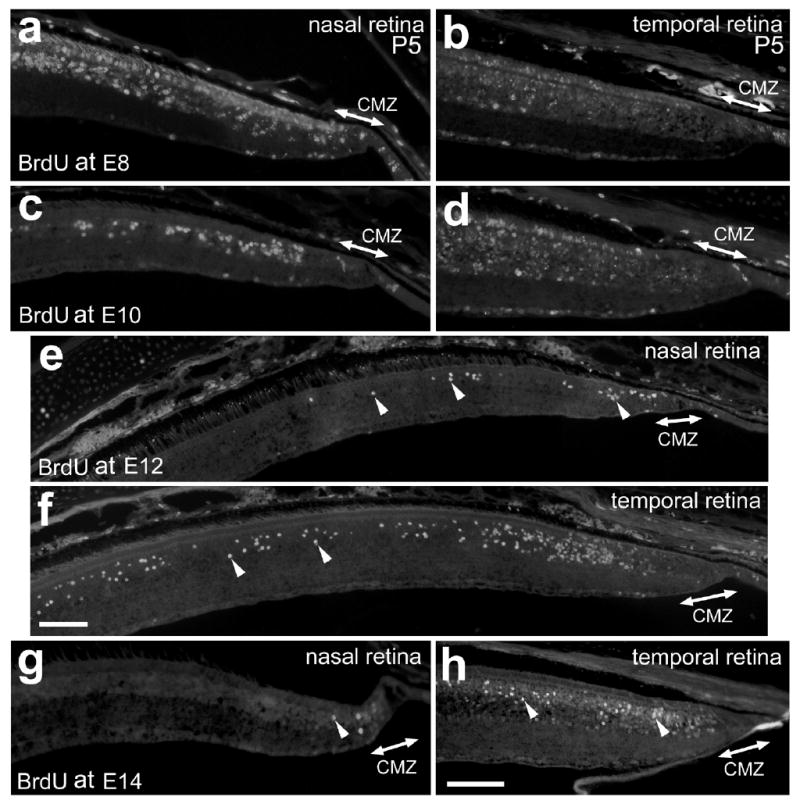
BrdU-birthdating of the cells in the far periphery of the retina. Vertical sections of the nasal (a, c, e and g) and temporal (b, d, f and h) retina and CMZ were labeled with antibodies to BrdU. BrdU was delivered to embryos at E8 (a and b), E10 (c and d), E12 (e and f) or E14 (g and h), and retinas were harvested at P5. Arrow-heads indicate BrdU-positive nuclei (e-h). The calibration bar (50 μm) in panel f applies to e and f, and the bar in h applies to a-d, g and h. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
In peripheral regions of the nasal retina, BrdU-treatment at E12 labeled a few nuclei scattered in the distal half of the INL (bipolar and/or Müller glia; Fig. 1e). Most of these cells were within 300 μm of the postnatal CMZ. In peripheral regions of the temporal retina, BrdU-treatment at E12 labeled numerous nuclei scattered in the distal half of the INL (bipolar and/or Müller glia; Fig. 1f); most of these cells were within 2000 μm of the postnatal CMZ. In addition, we observed a high density of BrdU-labeled cells in the ONL, INL and GCL within 400 μm of the temporal CMZ (Fig. 1f). This “band” of BrdU-labeled cells is reminiscent of the “bands” of H3-thymidine-labeled cells observed in the birthdating studies in frog retinas of Hollyfield over 30 years ago [23,24].
BrdU delivered at E14 failed to label photoreceptors in the ONL (Figs. 1g and h), indicating that the production of photoreceptors in the far peripheral regions of the retina is completed by E12 or E13. In the far periphery of the nasal retina, BrdU delivered at E14 labeled only a few cells that were usually found within 200 μm of the CMZ (Fig. 1g). In the temporal retina, BrdU delivered at E14 labeled numerous cells and nearly all of these cells were found in the distal INL and a few were scattered in the proximal INL (Fig. 1h). Regardless of when the BrdU was delivered to the embryo, we rarely observed BrdU-labeled cells within the CMZ (Fig. 1).
Proliferating cell nuclear antigen (PCNA) is known to be expressed by the progenitors in the postnatal chicken CMZ [14]. The PCNA antibody labels a subunit of DNA polymerase delta that is expressed highly in cells in the G1 and S phases of the cell cycle [28]. Thus, we assayed for the formation and patterning of the CMZ in the embryonic retina by using PCNA immunolabeling. As development proceeds, we observed a progressive restriction of PCNA-expression within far peripheral regions of the retina. At E12, all nuclei within the far peripheral retina, presumptive CMZ and presumptive NPE were immunoreactive for PCNA (Fig. 2a and b). Four days later at E16 there was a significant reduction in the region of cells that were PCNA-positive (Fig. 2c). Between 500 and 1000 μm away from the CMZ, PCNA-immunoreactivity was observed in fusiform nuclei near the middle of the INL (Figs. 2c and c′). These nuclei were likely those of differentiating Müller glia. Within 500 μm of the CMZ, we observed numerous PCNA-positive nuclei scattered across distal layers of the retina and these cells were concentrated at the peripheral edge of the retina (Figs. 2c and c″). In addition, we observed PCNA-immunoreactivity in pigmented cells that were distal to the CMZ and the NPE of the ciliary body (Figs. 2c and c″). At P3 we found PCNA-positive cells that were largely confined to the CMZ, consistent with previous reports[14]. In addition to cells within the CMZ, we found PCNA-immunoreactivity in the nuclei of NPE cells and pigmented cells overlying the NPE of the pars plana (Fig. 2d). Further, PCNA-immunoreactivity was observed in the nuclei of presumptive Müller glia, with fusiform nuclei in the middle of the INL; this immunoreactivity decreased in intensity with increasing distance from the CMZ (Fig. 2d).
Figure 2.
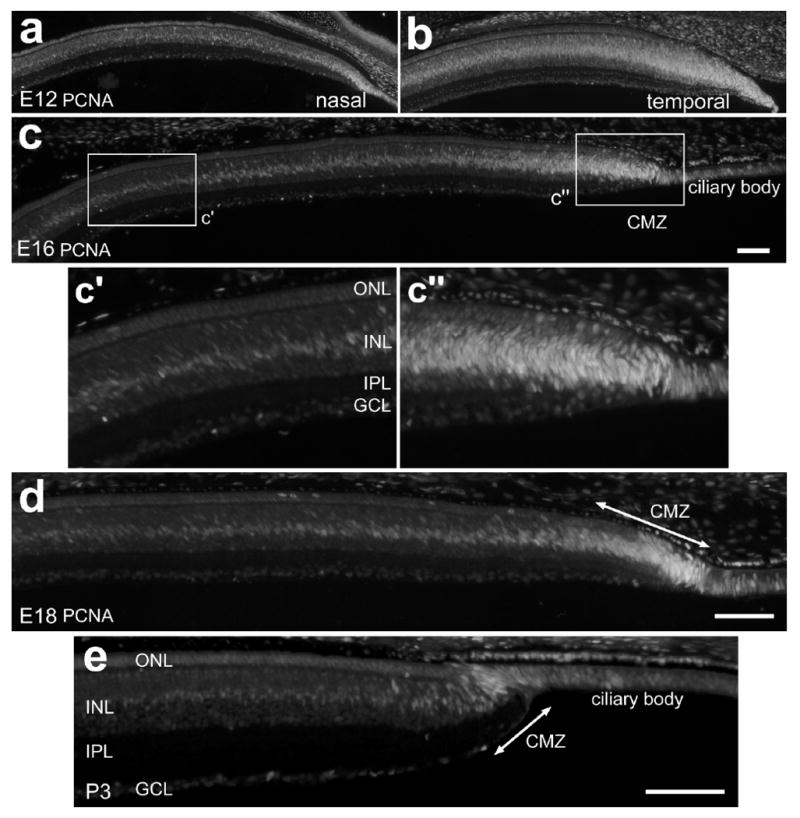
PCNA-expressing cells are gradually confined to the CMZ during late stages of embryonic development. Vertical sections of the peripheral retina were labeled with antibodies to PCNA. Tissues were obtained from animals at E12 (a and b), E16 (c), E18 (d) and P3 (e). The calibration bar (50 μm) in panel c applies to a-c, the bar in d applies to d alone, and the bar in panel e applies to e alone. The boxed-out areas in panel c are enlarged 2-fold in the underlying panels. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
Transitin-expression is gradually confined to the CMZ during late stages of embryonic development
The nestin-related intermediate filament transitin is expressed by neural progenitors as well as differentiating or reactive Müller glia in the embryonic and postnatal chicken retina [5]. We have shown previously that transitin-expression is gradually down-regulated by post-mitotic Müller glia in central regions of the retina from E12 to E18 and becomes restricted to peripheral retina sometime shortly before hatching, at E21 [5]. To determine exactly when transitin expression becomes confined to a CMZ, we looked at its distribution at two time points late in embryonic development. At E16, widespread distribution of transitin persists in the temporal retina and ciliary body (Fig. 3a); with immunoreactivity in vertically oriented processes that span all layers in the peripheral retina (Fig. 3a′). Transitin-immunoreactivity was more intense in the presumptive CMZ than in peripheral regions of the retina at E16 (Fig. 3a and a″). In peripheral regions of the nasal retina, transitin is restricted to filamentous structures in the GCL and NFL (Figs. 3b and b′). These structures likely were the endfeet of differentiating Müller glia. At E16, intense transitin-immunoreactivity persists across the CMZ and ciliary body (Figs. 3b and b″). Within a short span of time, between E16 and E18, transitin-immunoreactivity declines in peripheral regions of the temporal retina (Figs. 2c and c′) and is almost completely absent from peripheral regions of the nasal retina (Figs. 2d and d′). In addition, there is further restriction of transitin-immunoreactivity to the far periphery of the temporal retina and to the CMZ (Figs. 3c, c″, d and d″). At E18, transitin-expression in the nasal peripheral retina looks very similar to that seen at P3; persisting only in the CMZ (Figs. 3e and f). Consistent with patterns of PCNA-expression in the peripheral retina, we find that there is a gradual spatial restriction of transitin-positive progenitors to the far peripheral edge of the retina and into the CMZ during the last quarter of embryonic development.
Figure 3.
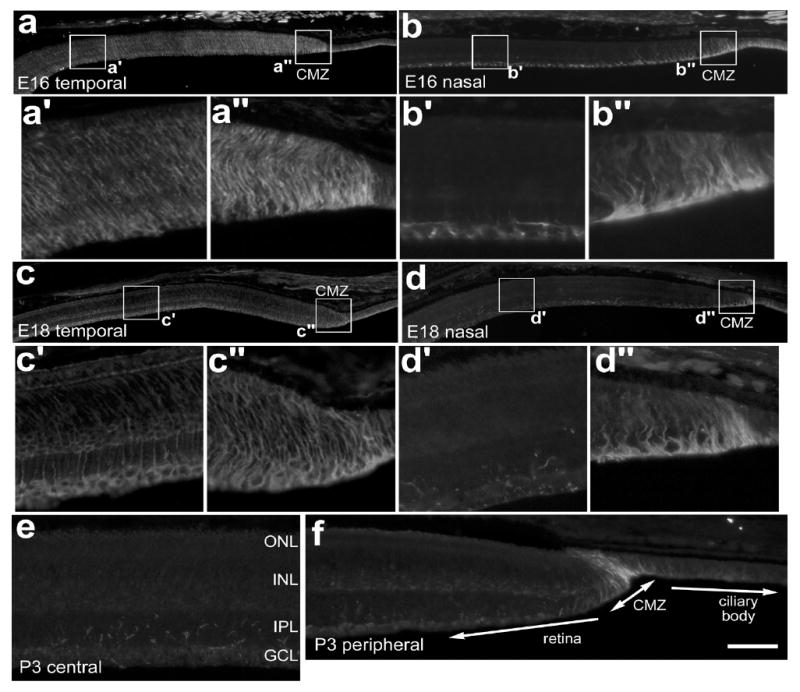
Transitin-expression is gradually confined to the CMZ during late stages of embryonic development. Tissues were obtained from animals at E16 (a and b), E18 (c and d), and P3 (e,f). Vertical sections of the retina were labeled with antibodies to transitin. Photomicrographs were taken of central (a′,b′,c′,d′), and far peripheral regions of the retina (a″,b″,c″,d″) where the presumptive or well-defined circumferential marginal zone is present. The calibration bar (50 μm) in panel d applies to panels a, b, c and d, and the bar in f applies to e and f. The boxed-out areas in panels a, b, c and d are enlarged 5-fold in the underlying panels. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
N-cadherin is gradually excluded from the neural retina and becomes confined to the CMZ and NPE of the ciliary body
The calcium-dependent adhesion molecule N-cadherin is known to be diffusely distributed on plasma membranes of retinal stem cells and progenitors in the CMZ and ciliary body, as well as on neuroepithelial progenitors in the zebrafish retinal primordium [29,30,38]. In the chick embryo, Wohrn et al [41] have shown a gradual down-regulation of N-cadherin expression in the central retina through development. Given that N-cadherin is expressed by progenitor cells, we wanted to determine whether it is present in the progenitor-rich CMZ in late stages of retinal development.
In the E16 peripheral retina, N-cadherin-immunoreactivity is restricted to the OPL and the distal ONL adjacent to the outer limiting membrane with very faint immunoreactivity in the IPL (Figs. 4a and a′). However, there is intense N-cadherin-immunoreactivity within the far periphery of the retina, including the presumptive CMZ and the NPE of the ciliary body (Figs. 4a and a″). At E18, N-cadherin-immunoreactivity is diminished in the peripheral distal retina compared to that seen at E16 (Figs. 4a′, a″, b and b″). However, N-cadherin-immunoreactivity in the CMZ and NPE of the ciliary body continues to be high (Fig. 4b″). In P3 eyes, N-cadherin-expression is absent from the far peripheral regions of the retina but continues to be maintained in the CMZ and NPE of the ciliary body (Fig 4c and f). This pattern of expression is maintained until at least P15 (data not shown). Taken together, these results indicate that spatial restriction of N-cadherin to the CMZ and NPE of the ciliary body occurs gradually during late stages of embryonic development and persists into postnatal development.
Figure 4.
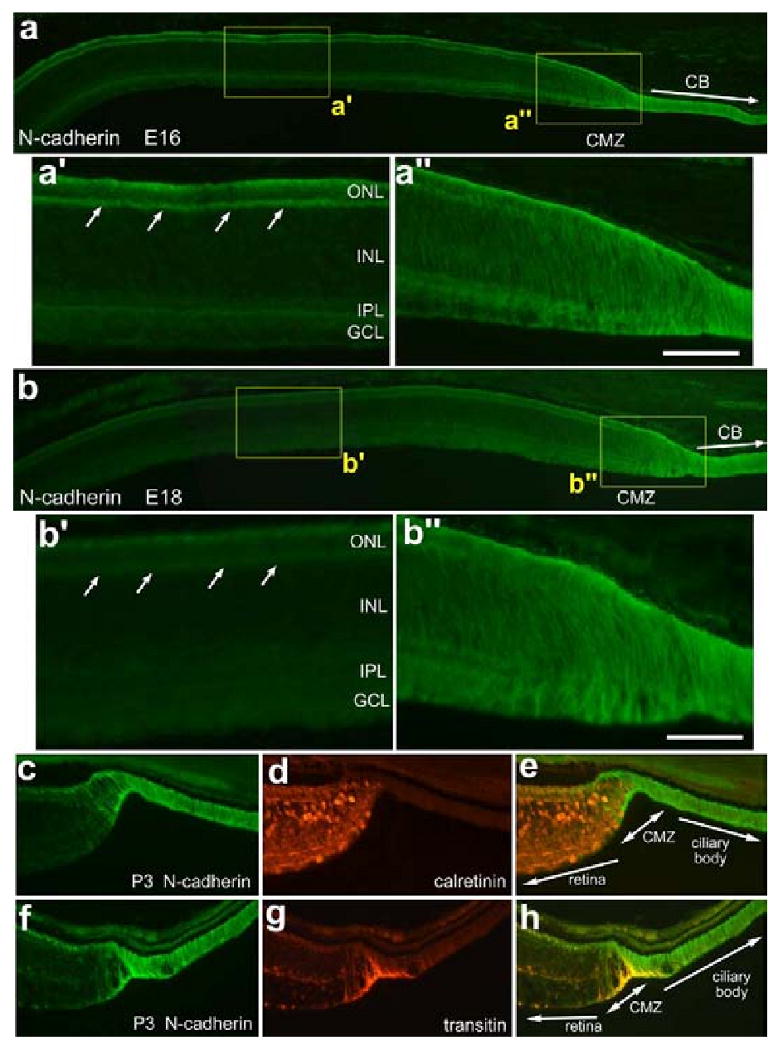
N-cadherin is highly expressed in the far peripheral retina, CMZ and ciliary body of both developing and postnatal retina and gets restricted over time in far peripheral regions of the retina. Tissues were obtained from animals at E16 (a), E18 (b), and P3 (c-h). Vertical sections of the retina were labeled with antibodies to N-cadherin (a and b) and calretinin (d) or transitin (g). Photomicrographs were taken of central (a′, b′), and far peripheral regions of the retina (a″,b″,c-h) where the CMZ is forming. The calibration bar (50 μm) in panel a″ applies to a′ and a″, the bar in b applies to a and b, the bar in b″ applies to b′ and b″, and the bar in h applies to c-h. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
To study N-cadherin expression relative to mature neurons, P3 tissue sections were labeled with antibodies to N-cadherin and calretinin, a calcium-binding protein present in the cytoplasm and neurites of horizontal, amacrine and ganglion cells [6,11]. We observed a mutually exclusive distribution of immunoreactivities, with calretinin-positive cells found immediately posterior or adjacent to the CMZ, while N-cadherin positive cells remain confined to the CMZ and the NPE of the ciliary body (Figs. 4c - e). Further, we observed N-cadherin-positive processes that wrap around the calretinin-positive neurons adjacent to the CMZ (Figs. 4c - e). The processes that were mingled among the neurons at the peripheral edge of the retina may have been those of differentiating Müller glia. At P3, there is a distinct overlap of transitin and N-cadherin immunoreactivity in the CMZ (Figs. 4f - h), clearly demarcating the CMZ and the transition from neural retina to the NPE of the ciliary body. These findings suggest that transitin- and N-cadherin-positive cells are involved in patterning the CMZ at late stages of embryonic retinal development despite having differing temporal and spatial expression patterns.
Neuronal differentiation is slowed in retinal regions that are adjacent to the CMZ
Early markers of neuronal differentiation are expressed by cells directly adjacent to the embryonic and postnatal CMZ. To identify the onset of neuronal differentiation we used markers that are known to be expressed soon after exit from the cell cycle. In the chick retina, the formation of cone photoreceptors may begin as early as E6 with onset of visinin-expression [2]. Calretinin is expressed by amacrine, horizontal and ganglion cells early during development, shortly after these cells exit the cycle and well before morphological differentiation is complete [6]. During early stages of retinal development in the chick embryo, HuD is expressed shortly after the terminal mitosis of amacrine and ganglion cells as these cells migrate into inner layers of the retina [12]. Not surprisingly, we found that visinin, calretinin and HuD are expressed by neurons that are found directly adjacent to the CMZ at E16 and P3 (Fig. 5). At the periphery of the retina, however, many visinin-positive cells had the morphology of immature photoreceptors with short outer segments and without distinct axon terminals (Figs. 5a, d and e). In addition, we found that many bipolar cells in peripheral regions of P3 retina were weakly immunoreactive for visinin (Figs. 5b and c), suggesting that these cells were still in the process of differentiating. Visinin is transiently expressed at low levels by a few subtypes of bipolar and amacrine cells in central regions of the embryonic chick retina as they differentiate (unpublished observations). By making consecutive daily intraocular injections of BrdU from P0 through P4 we failed to find evidence of newly generated photoreceptors, consistent with previous reports [14]. This finding is in agreement with the findings of our birthdating studies indicating that the generation of photoreceptors is completed in the temporal retina by E13.
Figure 5.
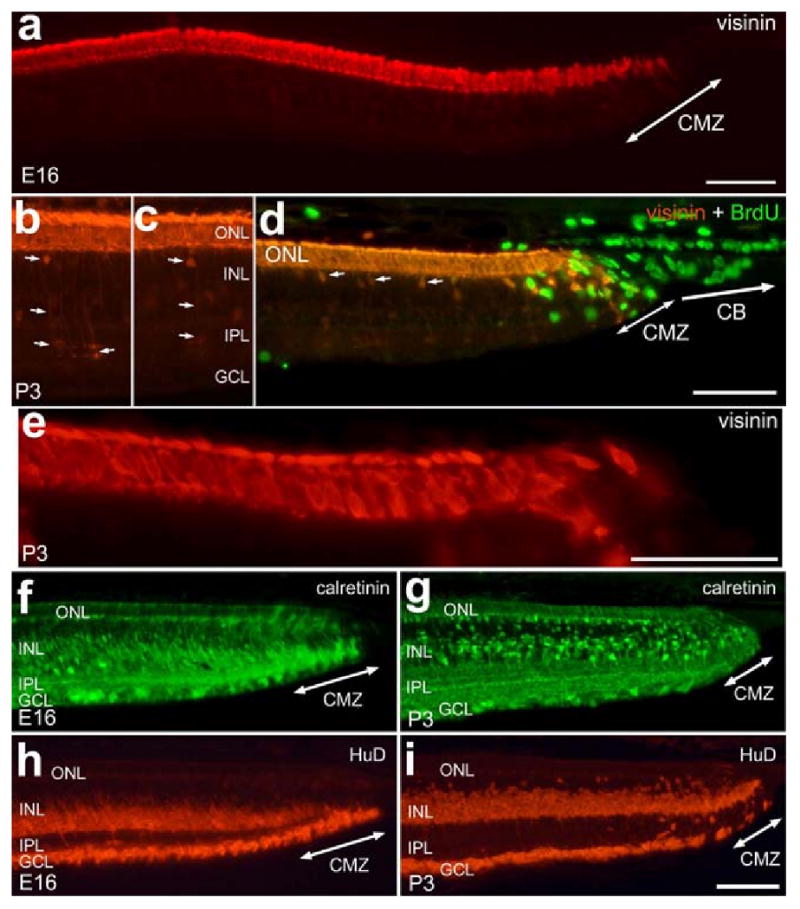
Early markers of neuronal differentiation are expressed by cells directly adjacent to the embryonic and postnatal CMZ. Tissues were obtained from animals at E16 (a, f and h) and P3 (b, c, d and e). Vertical sections of the retina were labeled with antibodies to visinin (red; a-e), BrdU (green; d), calretinin (green; f and g), and HuD (red; h and i). Tissues were obtained from chicks at E16 (a, f and h) or P3 (b-e, g and i). Arrows in b, c and d indicate visinin-positive bipolar cells. The calibration bar (50 μm) in panel a applies to a alone, the bar in d applies to b-d, the bar in e applies to e alone and the bar in i applies to f-i. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
Similar to the visinin-immunoreactive photoreceptors, the calretinin- and HuD-immunoreactive cells in peripheral regions of the retina had immature morphology with oblong somata and poorly defined neurites and cell bodies (Figs. 5f - i). In the E16 retina, the region of immature calretinin-expressing cells extended about 800 μm into the retina away from the CMZ (Fig. 5f). In the P3 retina, by comparison, the region of immature calretinin-immunoreactive cells extended only about 200 μm away from the CMZ (compare Figs. 5f and g). Similar to the pattern of calretinin-immunolabeling, HuD was expressed by neurons that extended into the retina at E16 while remaining directly adjacent to the CMZ at P3 (Figs. 5h and i).
To confirm that the far periphery of the retina remains immature in the postnatal retina we assayed for the expression of synaptophysin. Synaptophysin is a glycoprotein present in the pre-synaptic vesicles of most neurons [1]. In the developing chick retina immunoreactivity for synaptophysin is first detected at E7 in the IPL [21]. Synaptophysin has a diffuse distribution during early development and becomes confined to discrete lamina as development proceeds, indicating a gradual maturation of synapses within the plexiform layers of the retina. In the P3 eye, the CMZ is completely void of synaptophysin immunoreactivity (Fig. 6a and a″). Immunoreactivity for synaptophysin begins in the IPL at about 200 μm into the retina and gradually increases with increasing distance from the CMZ (Figs. 6a and 6b). Immunoreactivity for synaptophysin in the IPL is relatively diffuse within 800 μm of the CMZ, and gradually becomes concentrated in to discrete laminae (Fig. 6b). By comparison, synaptophysin immunoreactivity in the OPL does not appear until about 600 μm into the retina and gradually increases in the outer lamina of the OPL with increasing distance from the CMZ (Fig. 6a and a′). These findings suggest that in far peripheral regions of the retina, the synaptic connections in the IPL mature more rapidly than those in the OPL.
Figure 6.
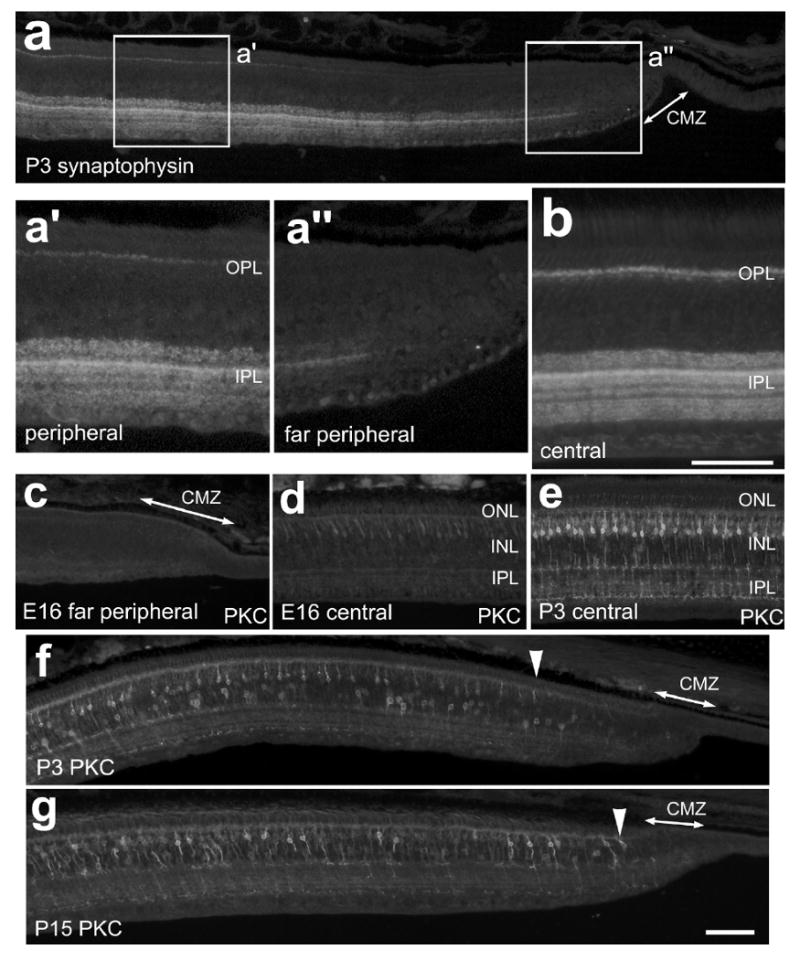
Synaptophysin and Protein Kinase C are expressed by neurons at increasing levels with increasing distance away from the CMZ. Tissues were obtained from animals at E16 (c and d), P3 (a, b, e and f) and P15 (g). Vertical sections of the peripheral retina and CMZ were labeled with antibodies to synaptophysin (a-b) or PKC (c-g). Panels f and g; arrow-heads indicate the onset of PKC expression in P3 and P15 retinas. The calibration bar (50 μm) in panel g applies to panels a, c, d, e, f and g. The boxed-out areas in panel a are enlarged 2-fold in the underlying panels. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
To better characterize the gradual maturation of retinal neurons in far peripheral regions of the retina, we applied antibodies to PKC, calbindin and red/green opsin; markers that are known to be expressed by differentiating neurons during late stages of development. In the chicken retina, PKC is known to be expressed by many bipolar cells in the distal INL, as well as a few types of amacrine cells in the proximal INL [17]. In the embryonic chick retina, PKC-immunoreactivity first appears in presumptive amacrine cells at about E9 and in differentiating bipolar cells at about E16 [3]. Consistent with previous reports, we did not detect immunoreactivity for PKC in central regions of the embryonic retina until E16 (Fig. 6d), about 8 days after these cells are generated [36]. At E16 there was no expression of PKC within 2 mm of the CMZ (Fig. 6c). In temporal regions of the P3 retina, the onset of PKC expression occurred at about 300 μm from the CMZ and steadily appeared in increasing numbers of bipolar cells with increasing distance from the CMZ (Fig. 6f). By comparison, nasal regions of the retina contained PKC-immunoreactive bipolar cells within 50 μm of the CMZ (data not shown). In temporal regions of the P15 retina, we found PKC-immunoreactive bipolar cells within 50 μm of the CMZ (Fig. 6g). These findings suggest a gradual maturation of PKC-positive bipolar cells in far peripheral regions of the temporal retina.
Calbindin is known to be expressed during the late stages of embryonic development in photoreceptors and bipolar cells [6]. Similarly, opsin-expression in photoreceptors begins at E15, about 10 days after terminal mitosis [2]. Consistent with these prior reports, we observed low levels of expression of calbindin and opsin in photoreceptors in central regions of the E16 chick retina (Figs. 7a and a′). By contrast, we failed to find immunoreactivity for calbindin or opsin in peripheral regions of E16 retina within 2-3 mm of the CMZ (Fig. 7b). At P3, we failed to find calbindin or opsin expression in far peripheral photoreceptors within approximately 300 μm of the temporal CMZ (Fig. 7c and c″). In nasal P3 retina, calbindin and opsin were expressed within 50 μm of the CMZ (data not shown). The “gap” in calbindin and opsin expression is decreased to less than 100 μm between P3 and P15 (Figs. 7e and e′), unlike visinin-immunoreactivity which remains in photoreceptors that are found adjacent to the CMZ at P15 (data not shown).
Figure 7.
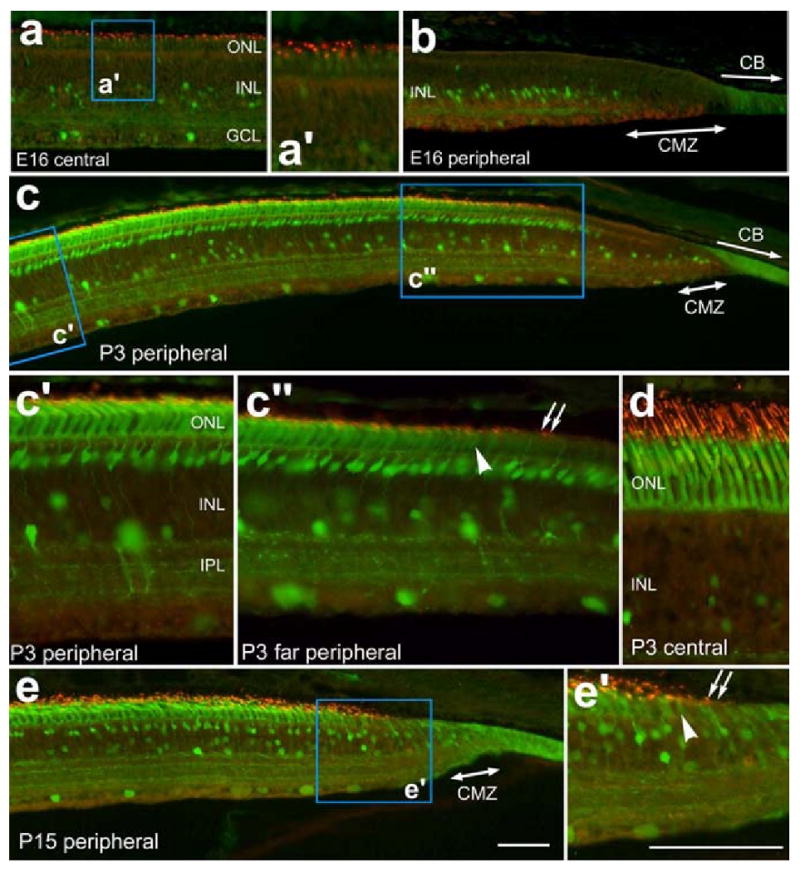
Photoreceptors and calbindin-expressing neurons remain immature in far peripheral regions of the temporal retina. Vertical sections of the retina were labeled with antibodies to calbindin (green) and red/green opsin (red). Tissues were obtained from animals at E16 (a, b), P3 (c, d) and P15 (e). Panels c″ and e′; arrow-heads indicate the onset of calbindin expression while arrows indicate the onset of red/green opsin expression in immature photoreceptors of the peripheral retina. The calibration bar (50) μm in panel e applies to panels a, b, c and e and the bar in e′ applies to a′, c′, c″, d and e′. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone.
Discussion
We report here that progenitors gradually become confined to the CMZ during late stages of embryonic development, between E16 and the time of hatching, at about E21. Proteins expressed by progenitors, such as PCNA, transitin and N-cadherin, are present in broad domains in peripheral regions of the late embryonic retina and become reduced during the last third of retinal development. For example, the zone of progenitors in the temporal retina is about 300 μm in diameter at E16, and over the following 8 days of development, this zone of progenitors is reduced to about 50 μm in diameter in the postnatal (P3) retina.
We found that the nasal CMZ becomes spatially defined several days before the temporal CMZ. The progenitor markers PCNA, transitin and N-cadherin are confined to a narrow region of CMZ in the nasal retina several days before these markers become restricted to the CMZ in the temporal retina. BrdU-birthdating studies indicate that progenitors in CMZ proliferate and add new neurons to the edge of the retina through late stages of development. Numbers of BrdU-labeled cells in the nasal retina are reduced at E12 and E14, when significantly more cells are BrdU-labeled in temporal regions of the retina. These studies indicate that cells in the far peripheral regions of the nasal retina are generated before those found in the far peripheral regions of the temporal retina. In line with these findings, we have reported previously that numbers of proliferating progenitors in the temporal CMZ are twice as abundant as those in the nasal CMZ [14]. We failed to observe significant numbers of BrdU-labeled cells within the postnatal CMZ when the BrdU was applied between E8 and E14 (see Fig. 1). Instead, we found BrdU-labeled cells within far peripheral regions of the retina, where postmitotic neurons reside. The absence of BrdU-labeled cells within the CMZ likely resulted from continued proliferation of CMZ progenitors and dilution of BrdU that was applied at or before E14.
We found that retinal neurons in far peripheral regions of the postnatal retina differentiate more slowly that those generated during embryonic development in the central retina. Figure 8 is schematic diagram illustrating the regions of the far peripheral postnatal retina where different neuronal markers are expressed, indicating different stages of neuronal differentiation and maturation. In temporal regions of the retina, our BrdU-birthdating studies indicate that the last photoreceptors are added to the retina between E12 and E13 (see Fig. 1). The photoreceptors in the far peripheral regions of the temporal retina do not mature and differentiate to express opsin or calbindin even at P15 (see Fig. 7), which is at least 24 days after terminal mitosis. Taken together, these findings indicate that photoreceptors at the far peripheral edge of the retina that are generated at around E12 remain undifferentiated for about 26 days after being generated. By comparison, cone photoreceptors in central regions of the retina are generated starting at about E5 [37], and begin to express opsins about 10 days later at E15 [2]. In line with these findings, a report in the primate retina has demonstrated that photoreceptors in far peripheral regions of the retina are maintained with an immature morphology into adulthood [9]. Furthermore, we found that synaptophysin-immunoreactivity in the OPL (indicating mature, functional synapses between photoreceptors and inner retinal neurons) was not present in the peripheral regions of the retina. The gradual onset of synaptophysin-expression with increasing distance from the CMZ further indicates that the photoreceptors differentiate slowly and eventually establish synaptic connections, perhaps after the onset of opsin- and calbindin-expression. Taken together these finding indicate that the photoreceptors that are generated near to the postnatal CMZ differentiate much more slowly than those generated in central regions of the retina, and these cells may be maintained in a relatively immature state.
Figure 8.
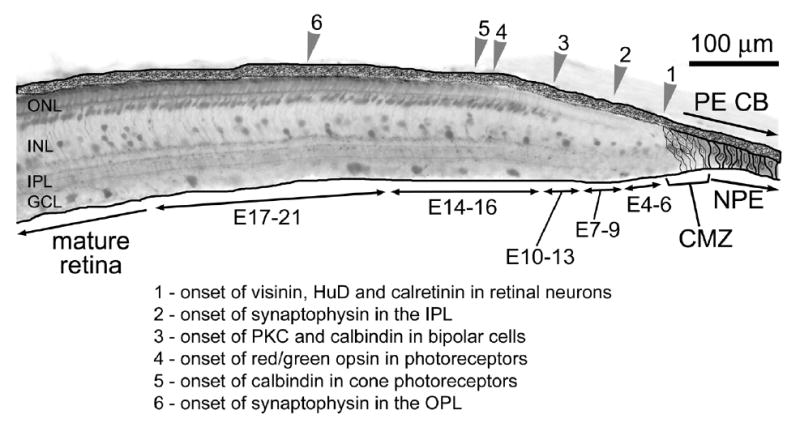
Schematic summary of the onset of different neuronal markers in the far peripheral retina. Abbreviations: ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; GCL, ganglion cell layer; CMZ, circumferential marginal zone; PE, pigmented epithelium; CB, ciliary body; NPE, non-pigmented epithelium; PKC, protein kinase C.
Similar to the stunted maturation of photoreceptors in far peripheral regions of the retina, the bipolar cells in the peripheral regions of the retina appear to differentiate much more slowly that those in central regions of the retina. For example, we found that the bipolar cells within 400 μm of the CMZ are generated between E12 and E14 (see Fig. 1f), and these cells express PKC sometime after P3, about 14 days after terminal mitosis (see Fig. 6g). By comparison, the bipolar cells in central regions of the retina are generated between E7 and E9 [36], and begin to express PKC at E16 (see Fig. 6d), about 8 days after terminal mitosis.
In addition to slowed differentiation of retinal neurons in far peripheral regions of the retina, the persistence of PCNA-immunoreactivity in the presumptive Müller glia near the CMZ, at late stages of embryonic development, also indicates retarded differentiation of this retinal cell type (see Fig. 2d) in the peripheral regions of the retina compared to the central retina.
Taken together these findings suggest that the microenvironment in the far peripheral retina serves to not only maintain a zone of progenitors, but also to maintain retinal neurons in an immature state. There are several possible explanations to account for the immature neurons in the far peripheral regions of the retina; (1) there are factors in the far peripheral retina that inhibit neuronal maturation, (2) there is a paucity of factors that stimulate the maturation of retinal neurons, (3) the late-born neurons in the retinal periphery are innately predisposed to mature slowly, or (4) a combination of these aforementioned possibilities.
Conclusions
Consistent with the report from Perron et al [35], we find a gradient of maturity in the far peripheral regions of the frog retina. In the chicken retina, we propose that this gradient of maturity extends well beyond the zone of progenitors within the CMZ and into neural retina.
The studies presented here indicate that: (1) there is a gradual spatial restriction of progenitors to form a CMZ during the late stages of embryonic development, (2) the CMZ at the nasal edge of the retina forms before the CMZ at the temporal edge of the retina, (3) the retinal neurons in far peripheral regions of the nasal retina differentiate and mature before those in far peripheral regions of the temporal retina, (4) the retinal neurons adjacent to the CMZ in far peripheral regions of the temporal retina remain immature and differentiate far more slowly compared to the neurons in central regions of the retina. The factors that keep retinal neurons in an immature state remain to be identified. The microenvironment at the periphery of the retina that promotes the persistence of a zone of retinal progenitors may also keep some types of neurons immature for extended periods of time.
Acknowledgments
We thank Dr. H. El-Hodiri for providing comments that contributed to the final form of this paper. We thank Dr. P. Henion for providing antibodies to transitin. The BrdU and N-cadherin antibodies developed by Drs S.J. Kaufman and K.A. Knudsen, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by grants provided by the National Institutes of Health (EY016043-01) and from the National Science Foundation (0413795).
References
- 1.Brandstatter JH, Lohrke S, Morgans CW, Wassle H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J Comp Neurol. 1996;370:1–10. doi: 10.1002/(SICI)1096-9861(19960617)370:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J Neurosci. 1996;16:1430–9. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminos E, Velasco A, Jarrin M, Aijon J, Lara JM. Protein kinase C-like immunoreactive cells in embryo and adult chicken retinas. Brain Res Dev Brain Res. 1999;118:227–30. doi: 10.1016/s0165-3806(99)00156-x. [DOI] [PubMed] [Google Scholar]
- 4.Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015–26. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- 5.Cole GJ, Lee JA. Immunocytochemical localization of a novel radial glial intermediate filament protein. Brain Res Dev Brain Res. 1997;101:225–38. doi: 10.1016/s0165-3806(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 6.Ellis JH, Richards DE, Rogers JH. Calretinin and calbindin in the retina of the developing chick. Cell Tissue Res. 1991;264:197–208. doi: 10.1007/BF00313956. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24:161–82. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–91. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- 9.Fischer AJ, Hendrickson A, Reh TA. Immunocytochemical characterization of cysts in the peripheral retina and pars plana of the adult primate. Invest Ophthalmol Vis Sci. 2001;42:3256–63. [PubMed] [Google Scholar]
- 10.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22:9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999;39:685–97. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 12.Fischer AJ, Omar G. Transitin, a nestin-related intermediate filament, is expressed by neural progenitors and can be induced in Muller glia in the chicken retina. J Comp Neurol. 2005;484:1–14. doi: 10.1002/cne.20406. [DOI] [PubMed] [Google Scholar]
- 13.Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25:10157–66. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- 15.Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 16.Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259:225–40. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 17.Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- 18.Fischer AJ, Skorupa D, Schonberg DL, Walton NA. Characterization of glucagon-expressing neurons in the chicken retina. J Comp Neurol. 2006;496:479–94. doi: 10.1002/cne.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer AJ, Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405:1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–72. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 21.Hering H, Kroger S. Formation of synaptic specializations in the inner plexiform layer of the developing chick retina. J Comp Neurol. 1996;375:393–405. doi: 10.1002/(SICI)1096-9861(19961118)375:3<393::AID-CNE4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23:183–94. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Hollyfield JG. Differential addition of cells to the retina in Rana pipiens tadpoles. Dev Biol. 1968;18:163–79. doi: 10.1016/0012-1606(68)90041-9. [DOI] [PubMed] [Google Scholar]
- 24.Hollyfield JG. Differential growth of the neural retina in Xenopus laevis larvae. Dev Biol. 1971;24:264–86. doi: 10.1016/0012-1606(71)90098-4. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MM, Phanhthourath C, Brees DK, McCabe CF, Cole GJ. Molecular characterization of EAP-300: a high molecular weight, embryonic polypeptide containing an amino acid repeat comprised of multiple leucine-zipper motifs. Brain Res Dev Brain Res. 1995;85:31–47. doi: 10.1016/0165-3806(94)00185-3. [DOI] [PubMed] [Google Scholar]
- 26.Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 27.Kubota R, McGuire C, Dierks B, Reh TA. Identification of ciliary epithelial-specific genes using subtractive libraries and cDNA arrays in the avian eye. Dev Dyn. 2004;229:529–40. doi: 10.1002/dvdy.20000. [DOI] [PubMed] [Google Scholar]
- 28.Kurki P, Ogata K, Tan EM. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for probing cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods. 1988;109:49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Babb SG, Novince ZM, Doedens AL, Marrs J, Raymond PA. Differential expression of cadherin-2 and cadherin-4 in the developing and adult zebrafish visual system. Vis Neurosci. 2001;18:923–33. [PubMed] [Google Scholar]
- 30.Liu Q, Londraville RL, Azodi E, Babb SG, Chiappini-Williamson C, Marrs JA, Raymond PA. Up-regulation of cadherin-2 and cadherin-4 in regenerating visual structures of adult zebrafish. Exp Neurol. 2002;177:396–406. doi: 10.1006/exnr.2002.8008. [DOI] [PubMed] [Google Scholar]
- 31.Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–55. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 32.Moshiri A, McGuire CR, Reh TA. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev Dyn. 2005 doi: 10.1002/dvdy.20299. [DOI] [PubMed] [Google Scholar]
- 33.Moshiri A, Reh TA. Persistent progenitors at the retinal margin of ptc+/- mice. J Neurosci. 2004;24:229–37. doi: 10.1523/JNEUROSCI.2980-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–36. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 35.Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- 36.Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 37.Prada F, Medina JI, Lopez-Gallardo M, Lopez R, Quesada A, Spira A, Prada C. Spatiotemporal gradients of differentiation of chick retina types I and II cholinergic cells: identification of a common postmitotic cell population. J Comp Neurol. 1999;410:457–66. [PubMed] [Google Scholar]
- 38.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58:296–305. doi: 10.1159/000057571. [DOI] [PubMed] [Google Scholar]
- 40.Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–20. [PubMed] [Google Scholar]
- 41.Wohrn JC, Puelles L, Nakagawa S, Takeichi M, Redies C. Cadherin expression in the retina and retinofugal pathways of the chicken embryo. J Comp Neurol. 1998;396:20–38. [PubMed] [Google Scholar]


