Abstract
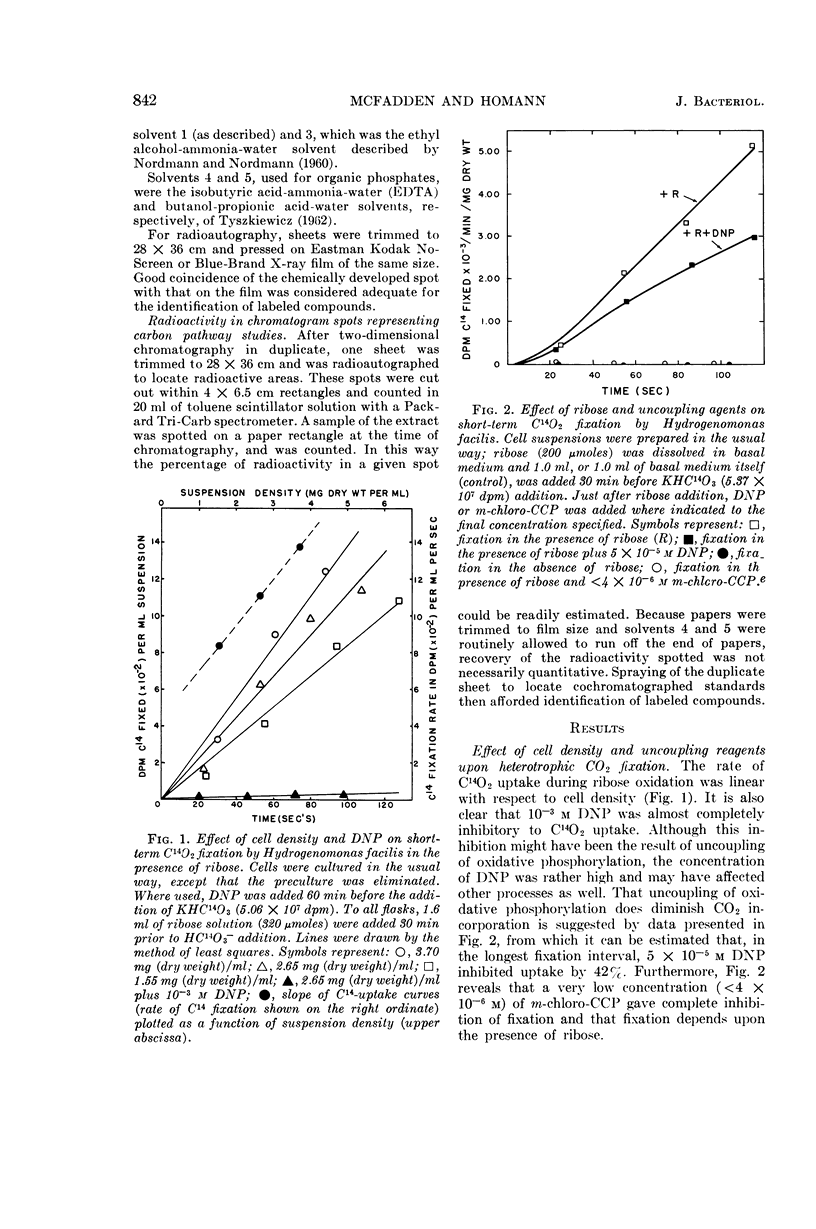
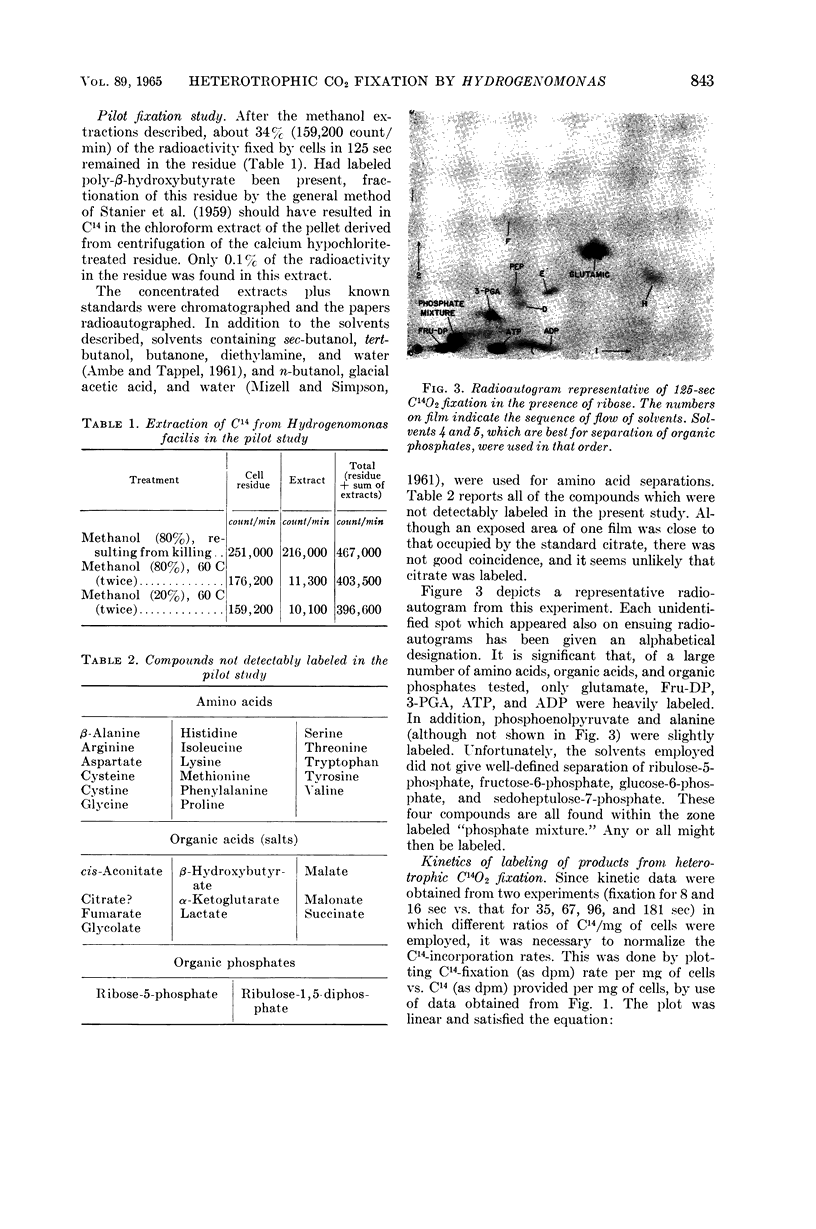
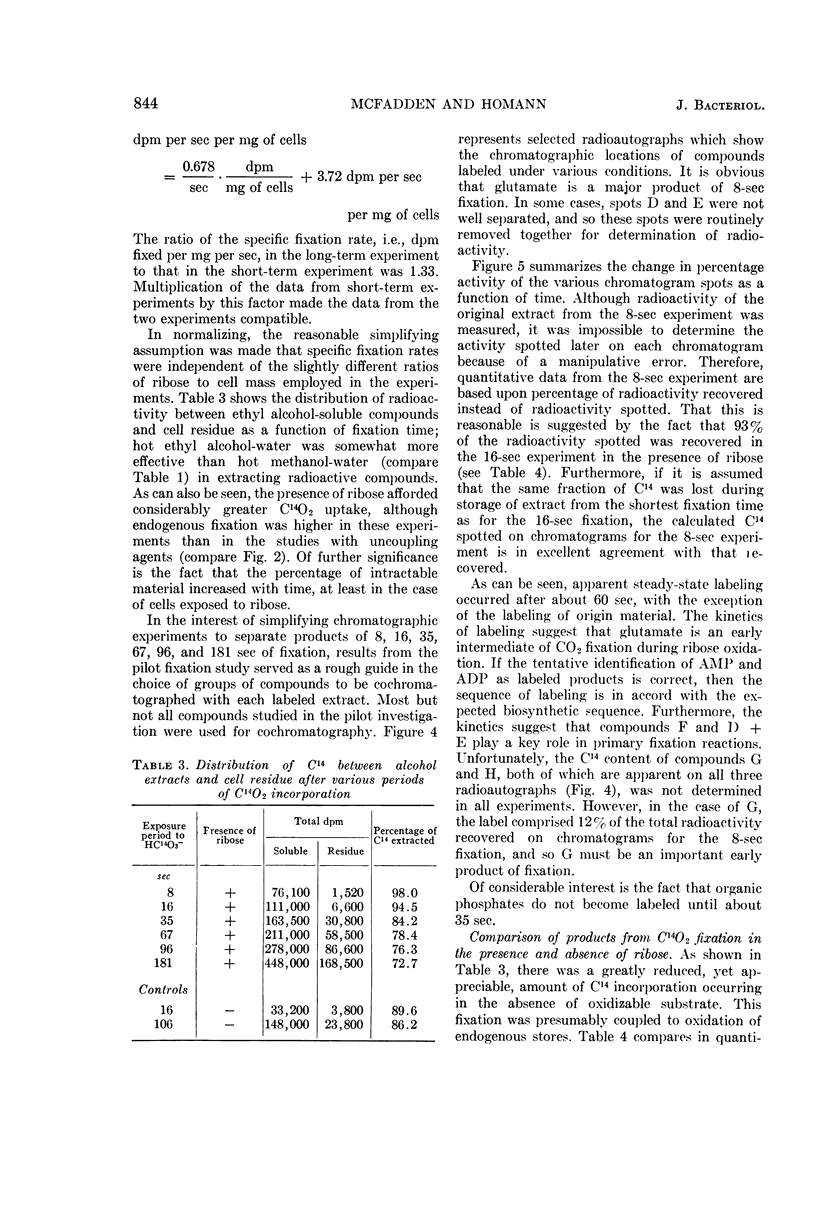
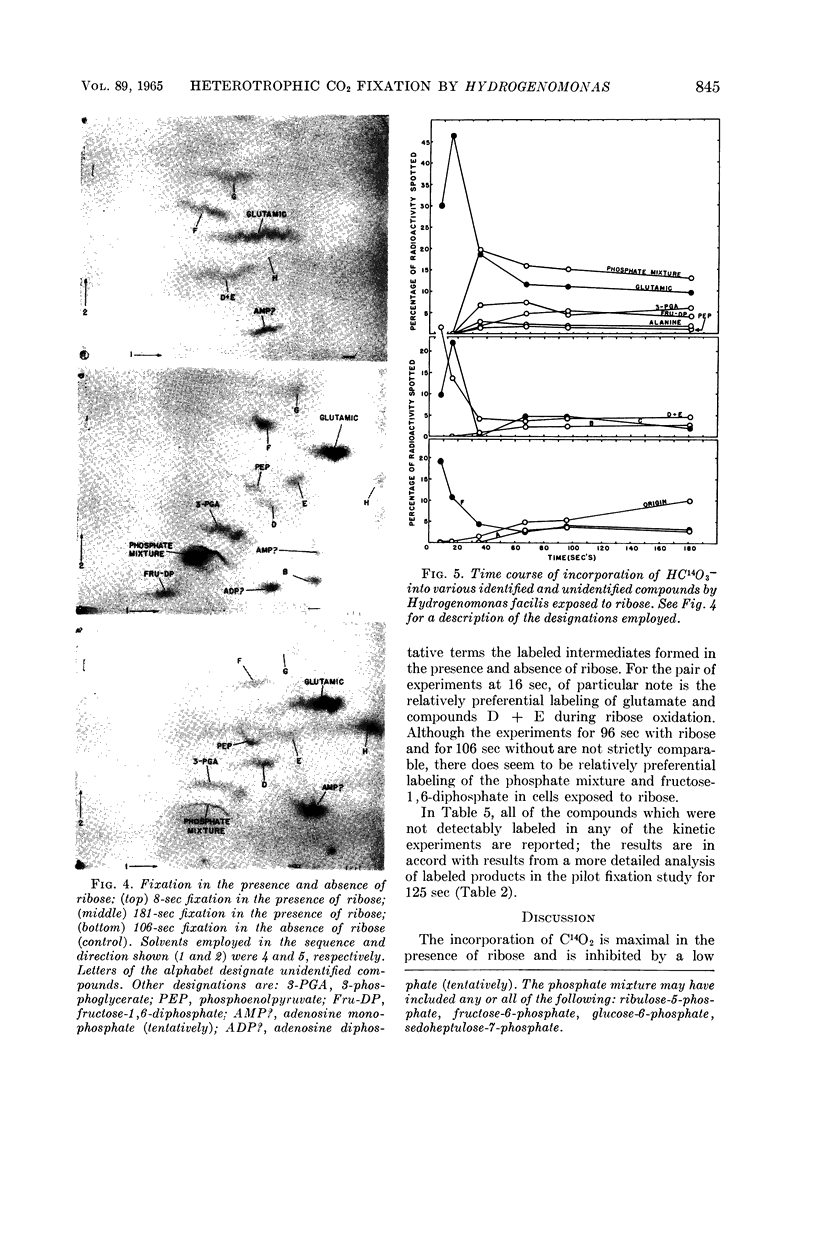
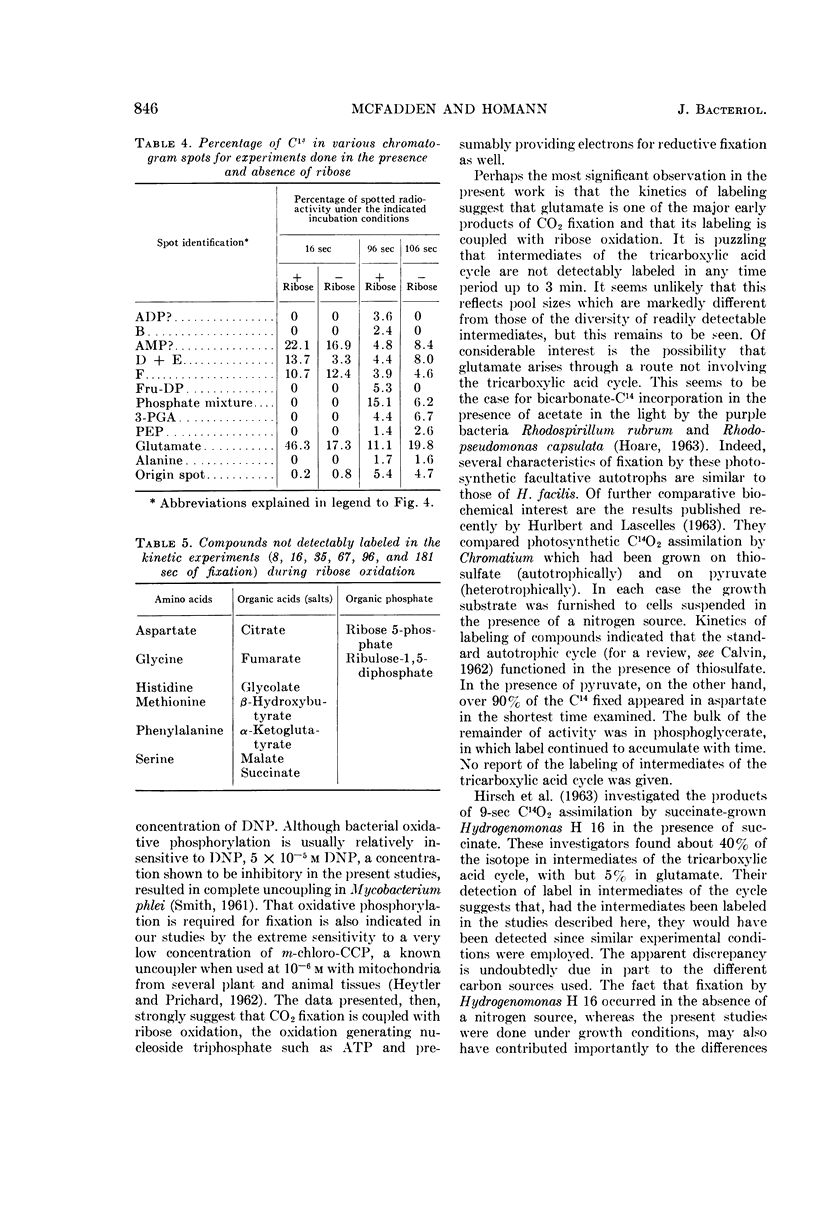
McFadden, B. A. (Washington State University, Pullman), and H. R. Homann. Characteristics and intermediates of short-term C14O2 incorporation during ribose oxidation by Hydrogenomonas facilis. J. Bacteriol. 89:839–847. 1965.—Ribose-grown cells of Hydrogenomonas facilis, which had been suspended in growth medium and were oxidizing ribose, were exposed to HC14O3− of high specific activity. The uptake was proportional to cell mass. Short-term uptake (less than 2 min) was completely inhibited by 10−3m 2,4-dinitrophenol (DNP) or by <4 × 10−6mm-chlorocarbonyl cyanide phenylhydrazone, and to the extent of 42% by 5 × 10−5m DNP. The following observations were made in kinetic studies (8, 16, 35, 67, 96, and 181 sec) of fixation in the presence of ribose. Glutamate was extensively labeled in periods up to 3 min. It was one of the major early products, containing 30% of the label at 8 sec. The sugar phosphate fraction was not detectably labeled at 8 or 16 sec, but its C14-content increased rapidly to 27% at 35 sec and then slowly decreased. Label in phosphoglycerate, phosphoenolpyruvate, and alanine did not appear until 35 sec, and did not exceed about 7, 2, and 3%, respectively, of the total extracted radioactivity. Adenosine triphosphate and adenosine diphosphate were heavily labeled after fixation in a pilot study for 125 sec. Although considerable radioactivity incorporated during the pilot study was intractable by the extraction procedure employed, virtually no C14 was found in the residue in poly-β-hydroxybutyric acid. A large number of amino acids and organic acids and some organic phosphates were not detectably labeled in any of the experiments. Omission of ribose greatly diminished incorporation, particularly into glutamate.
Full text
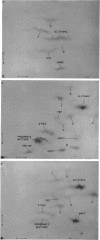
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., MCFADDEN B. A. The biochemistry of Hydrogenomonas. V. Factors affecting autotrophic fixation of carbon dioxide. Arch Biochem Biophys. 1957 Jan;66(1):16–22. doi: 10.1016/0003-9861(57)90533-7. [DOI] [PubMed] [Google Scholar]
- BERGMANN F. H., TOWNE J. C., BURRIS R. H. Assimilation of carbon dioxide by hydrogen bacteria. J Biol Chem. 1958 Jan;230(1):13–24. [PubMed] [Google Scholar]
- CALVIN M. The path of carbon in photosynthesis. Science. 1962 Mar 16;135(3507):879–889. doi: 10.1126/science.135.3507.879. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- HIRSCH P., GEORGIEV G., SCHLEGEL H. G. CO2-FIXIERUNG DURCH KNALLGASBAKTERIEN. III. AUTOTROPHE UND ORGANOTROPHE CO2-FIXIERUNG. Arch Mikrobiol. 1963 Jul 18;46:79–95. [PubMed] [Google Scholar]
- HOARE D. S. The photo-assimilation of acetate by Rhodospirillum rubrum. Biochem J. 1963 May;87:284–301. doi: 10.1042/bj0870284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. E., LASCELLES J. RIBULOSE DIPHOSPHATE CARBOXYLASE IN THIORHODACEAE. J Gen Microbiol. 1963 Dec;33:445–458. doi: 10.1099/00221287-33-3-445. [DOI] [PubMed] [Google Scholar]
- MCFADDEN B. A., HOMANN H. R. QUANTITATIVE STUDIES OF THE EFFECT OF ORGANIC SUBSTRATES AND 2,4-DINITROPHENOL ON HETEROTROPHIC CARBON DIOXIDE FIXATION IN HYDROGENOMONAS FACILIS. J Bacteriol. 1963 Nov;86:971–977. doi: 10.1128/jb.86.5.971-977.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFADDEN B. A. Some products of C1402 fixation by Hydrogenomonas facilis. J Bacteriol. 1959 Mar;77(3):339–343. doi: 10.1128/jb.77.3.339-343.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ A., BOVELL C., Jr Growth and hydrogenase activity of a new bacterium, Hydrogenomonas facilis. J Bacteriol. 1952 Jan;63(1):87–98. doi: 10.1128/jb.63.1.87-98.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYSZKIEWICZ E. An improved solvent system for the paper chromatography of phosphate esters. Anal Biochem. 1962 Feb;3:164–166. doi: 10.1016/0003-2697(62)90107-0. [DOI] [PubMed] [Google Scholar]
- WALKER D. G., WARREN F. L. Filter paper chromatography of phosphate esters. Biochem J. 1951 Jun;49(1):xxi–xxi. [PubMed] [Google Scholar]