Table 2.
Driving Forces, Rate Constants, and Kinetic Isotope Effects for Nitroxyl plus Hydroxylamine Reactions.a
| Reaction | Solvent | ΔG°b | kH (M−1 s−1)a | kD (M−1 s−1)a,c | kH/kDa,c | Ref. |
|---|---|---|---|---|---|---|
| 4-oxo-TEMPO• + TEMPO-Hd | MeCN | −0.9 ± 0.2 | 10 ± 1 | 0.44 ± 0.05 | 23 ± 3 | e |
| 4-oxo-TEMPO• + TEMPO-H | CH2Cl2 | −1.2 ± 0.2 | 48 ± 4 | 2.1 ± 0.3 | 23 ± 4 | e |
| 4-oxo-TEMPO• + TEMPO-H | CCl4 | – | 300 ± 30 | 17 ± 4 | 18 ± 5 | e |
| 4-oxo-TEMPO• + 4-MeO-TEMPO-H | MeCN | −0.6 ± 0.2 | 7.8 ± 0.7 | 0.37 ± 0.05 | 21 ± 3 | e |
| tBu2NO• + TEMPO-H | MeCN | 1.3 ± 0.2 | 1.9 ± 0.2 | 0.12 ± 0.02 | 16 ± 3 | e |
| tBu2NO• + TEMPO-H | CH2Cl2 | 1.4 ± 0.2 | 4.6 ± 0.4 | 0.35 ± 0.04 | 13 ± 2 | e |
| PINO• + 4Me-NHPIf | AcOH(D) | −0.4 ± 0.1 | 677 ± 24 | 61.3 ± 2.1 | 11.0 ± 0.5 | 18 |
| 4-Me-PINO• + NHPIf | AcOH(D) | 0.4 ± 0.1 | 354 ± 23 | 31.8 ± 2.0 | 11.1 ± 1.0 | 18 |
| tBu2NO• + tBu2NOH | CCl4 | 0 | 320 ± 40 | – | – | 13 |
| tBu2NO• + tBu2NOH | C6H5Cl | 0 | 240 ± 60 | – | – | 13 |
| tBu(Ar)NO• + tBu(Ar)NOHg | CCl4 | 0 | (2.0 ± 0.4) × 103 | (1.3 ± 0.2) × 103 | 1.5 ± 0.4 | 13 |
| tBu(Ar)NO• + tBu(Ar)NOHg | C6H5Cl | 0 | (5.2 ± 0.4) × 102 | – | – | 13 |
| tBu(Ar)NO• + tBu(Ar)NOHg | CH2Cl2 | 0 | < 20 | – | – | 13 |
| Ph2NO• + Ph2NOH | CCl4 | 0 | > 107 | – | – | 13 |
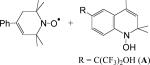 |
hexane | −0.2 ± 0.1 | (4.3 ± 0.2) × 104 | (2.5 ± 0.1) × 104 | 1.7 ± 0.1 | 14 |
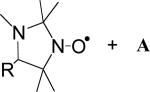 |
hexane | 2.0 ± 0.1 | (4.5 ± 0.2) × 103 | (2.9 ± 0.2) × 103 | 1.6 ± 0.1 | 14 |
| B + A (R = CPh3) | hexane | 0.9 ± 0.1 | (1.4 ± 0.1) × 104 | (7.3 ± 0.4) × 103 | 1.9 ± 0.2 | 14 |
| B (R = Ph) + A | hexane | 1.6 ± 0.1 | (8.6 ± 0.4) × 103 | (5.7 ± 0.3) × 103 | 1.5 ± 0.1 | 14 |
| TEMPO• + A | hexane | −0.6 ± 0.1 | (7.6 ± 0.4) × 104 | – | – | 14 |
| TEMPO• + A (R = CPh3) | hexane | −1.4 ± 0.1 | (1.5 ± 0.1) × 105 | – | – | 14 |
| 4-oxo-TEMPO• + A | hexane | −1.3 ± 0.1 | (6.4 ± 0.3) × 104 | – | – | 14 |
kcal mol−1.
Not corrected for the incomplete (98±1%) deuterium enrichment; the true kH/kD values are roughly a factor of two higher; see text.
Ref. 20.
This work.
PINO• = phthalimide N-oxyl radical, NHPI = N-hydroxyphthalimide.
Ar = 2,6-dimethoxyphenyl.
